Comparison of EFV vs MVC
MERIT Study: maraviroc vs efavirenz,�in combination with ZDV/3TC
Original article : J Infect Dis. 2010 Mar 15;201(6):803-13 - DA Cooper
Last update :
28/03/2014
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
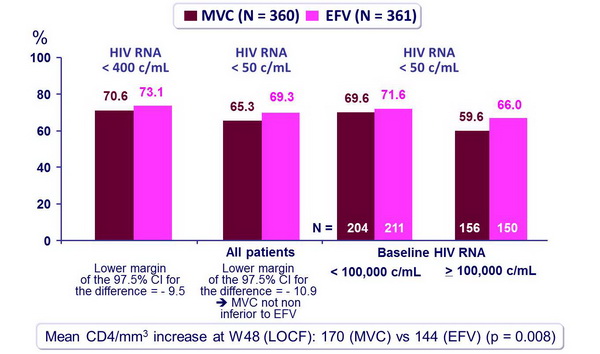
- MVC was not non inferior to EFV, when combined with ZDV/3TC
- Increase in CD4 count was significantly greater for MVC than for EFV
- There were more discontinuations for lack of efficacy in the MVC group
- When the screening samples were retested with the more sensitive Trofile assay, 15% of patients were found to have CXCR4 virus at screening
- In the post hoc reanalysis, excluding these patients
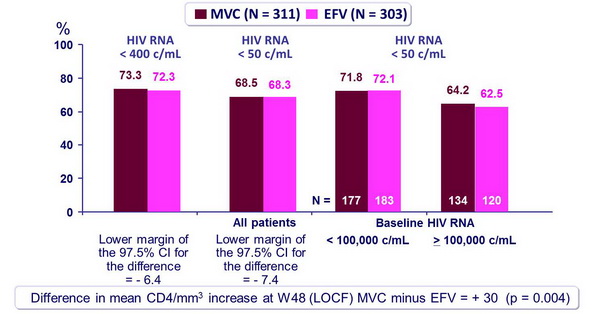
- MVC was non inferior to EFV, for proportion of HIV RNA < 50 c/mL at W48
- Virologic response rates were similar between MVC and EFV within each viral load stratum (HIV RNA < or > 100,000 c/mL)
- Response rate was higher for MVC in patients from Northern Hemisphere due to higher adverse event-related discontinuations for EFV
- Response rate was lower for MVC in patients from Southern Hemisphere, this result being driven by more black patients receiving MVC than EFV defaulting
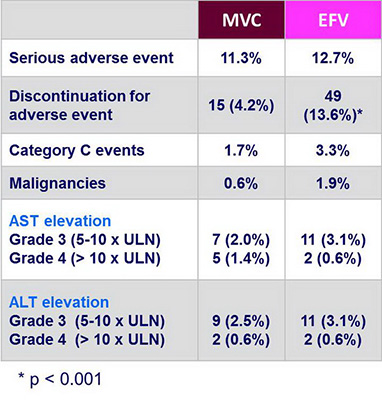
- MVC was associated with significantly fewer adverse event-related discontinuations than EFV and with fewer malignancies and category C events
- There were no difference between groups in the incidence of elevation in transaminases, and there were no unexpected safety findings
Design :
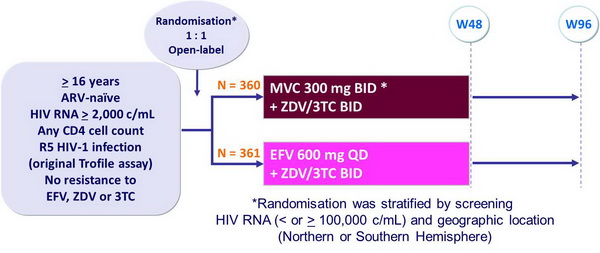
Objective :
- Non inferiority of MVC vs EFV: % HIV RNA < 400 c/mL and < 50 c/mL�(co-primary endpoints) at W48, by ITT analysis (lower margin of the 1-sided 97.5% CI for the difference = - 10%) [missing values were classified as non responses]
* A third arm with MVC 300 mg QD was discontinued for lack of efficacy at W16
Baseline characteristics and patient disposition :
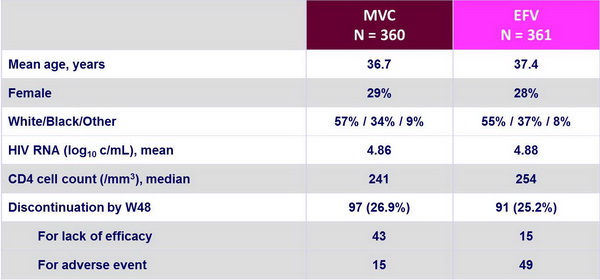
78% of black and 72% of female patients were in the Southern Hemisphere Screening HIV RNA > 100,000 c/mL: 45% in the Southern Hemisphere vs 38%�in the Northern Hemisphere
Response to treatment at week 48 (ITT) :
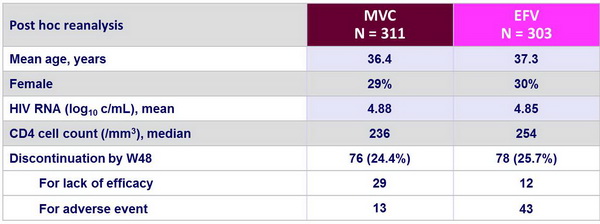
Post hoc reanalysis : A descriptive post hoc reanalysis was done for patients whose screening samples retrospectively retested as R5 by the current Trofile assay. The enhanced version of this assay has greater sensitivity for detecting minority CXCR4-using strains than the original Trofile assay. This led to exclude 107 �of the 721 original patients
Baseline characteristics and patient disposition (Post hoc reanalysis) :
Response to treatment at week 48 (ITT): post hoc reanalysis (exclusion of patients with non-R5 virus at screening by the enhanced Trofile assay) :
Safety (Original patient population) :
Resistance (Post hoc reanalysis) :
- Virologic failure by the TLOVR algorithm (HIV RNA > 50 c/mL):29
- MVC vs 13 EFV� MVC failures, N = 29
- Emergence of CXCR4-using virus, N = 9
- Emergence of R5 resistant to MVC, N = 4
- R5 virus without any resistance, N = 11
- Resistance to 3TC only, N = 5�
- EFV failures, N = 13
- Emergence of resistance to EFV, N = 9
- Resistance to 3TC only, N = 1
- No resistance, N = 3
 Back to Table of Contents
Back to Table of Contents



