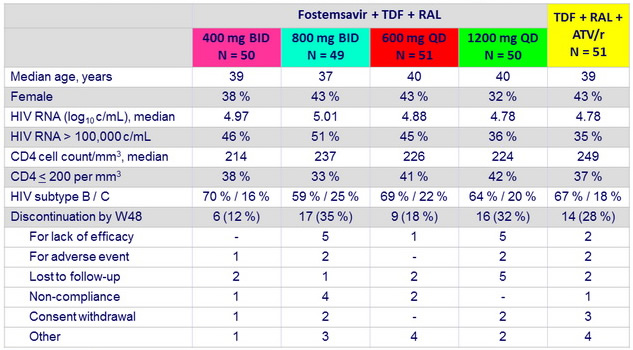
Baseline characteristics and patient disposition
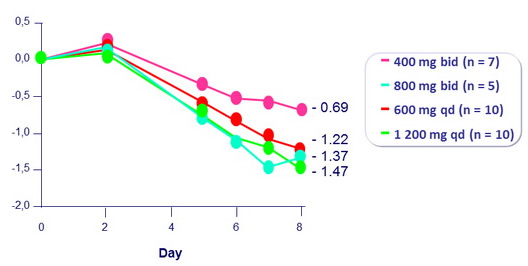
Fostemsavir 7 days of monotherapy
Mean change in HIV RNA from baseline (log 10 c/ml)
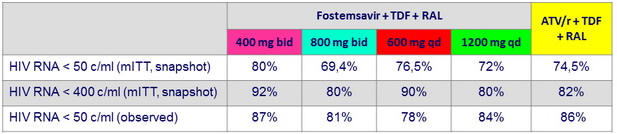
HIV RNA < 50 c/ mL or < 400 c/ mL at W24
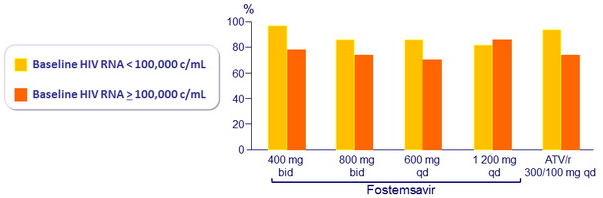
HIV RNA < 50 c/ml at W24 by baseline HIV RNA (observed )
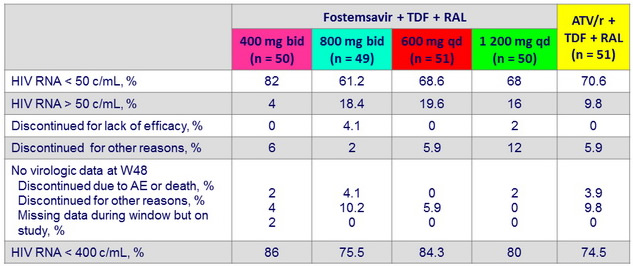
HIV RNA < 50 c/ mL or < 400 c/ mL at W48, mITT snapshot
HIV RNA < 50 c/ mL at W48, observed

Safety data through W48
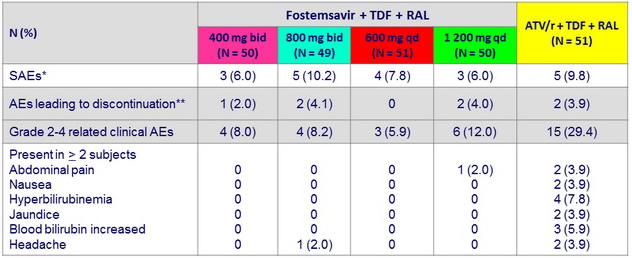
* Anal abscess, herpes encephalitis, overdose (3), extrapulmonary tuberculosis (2), herpes zoster , abdominal pain, myalgia ,
spontaneous abortion, acute renal failure , cellulitis (2), lymphangitis , chronic cholecystitis , back pain, pneumonia , pyelonephritis ,
diarrhea , cholelithiasis , migraine
** Illegal substance use, extrapulmonary tuberculosis (3), acute renal failure , abdominal distension, flatulence, nausea , jaundice ; 6/7 AE leading to discontinuation in first 24 weeks
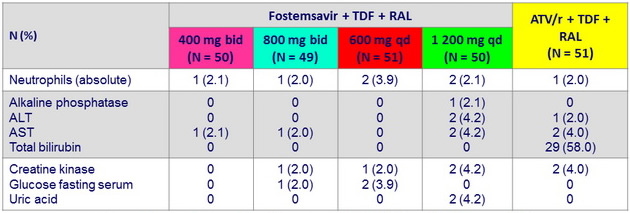
Grade 3-4 laboratory abnormalities (≥ 2 subjects )
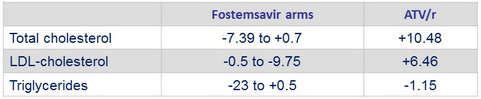
Mean change from baseline at W48 in fasting lipids , mg/ dL