Doravirine
(non nucleoside reverse transcriptase inhibitor)
Etude MK1439007 :
doravirine (DOR) + TDF/FTC vs EFV
+ TDF/FTC
Original article :
Morales-Ramirez JO, CROI 2014, Abs. 92LB, Gatell JM. HIV Drug Therapy 2014, Abs. O434
Last update :
02/06/2015
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
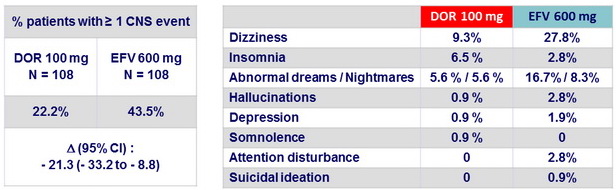
- In antiretroviral-naïve, HIV-1 infected subjects, DOR 100 mg qd + TDF/FTC had a lower rate of treatment-emergent CNS events by week 8 than EFV + TDF/FTC
- DOR 25 to 200 mg qd for 48 weeks
- had simialr virologic and immunologic efficacy to EFV
- with low rate of resistance mutation development
- and good safety and tolerability profile
- DOR 100 mg qd dose was selected for further development
Design
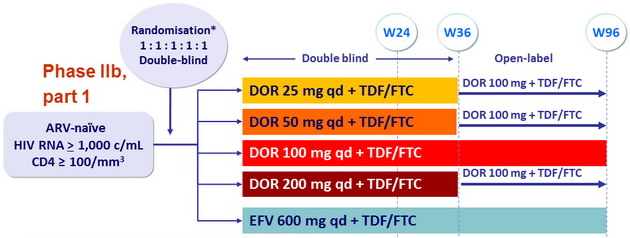
* Randomisation stratified on HIV RNA (> or ≤ 100,000 c/mL)
Objective
- Primary endpoints
- % HIV-1 RNA < 40 c/mL at W24 (estimation comparisons for DOR dose selection), ITT, NC=F
- Safety : general at W24, pre-specified CNS AEs by W8 and W24
Design
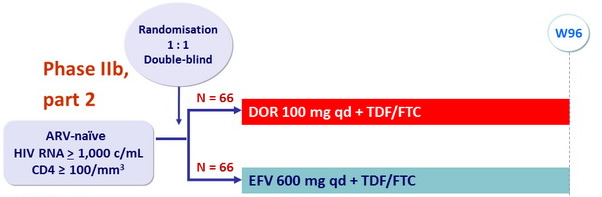
Objective
- CNS adverse events analysis, W8
- Parts 1 and 2 combined (DOR 100 mg vs EFV)
- Efficacy and safety analyses, W48 : part 1 only, W96 : parts 1 and 2
- % with HIV RNA < 40 c/ mL , < 200 c/ mL , NC=F approach for missing data
- Change from baseline in CD4 cell count, observed failure approach
- Safety endpoints : adverse events, laboratory parameters
Baseline characteristics and patient disposition (Part 1)
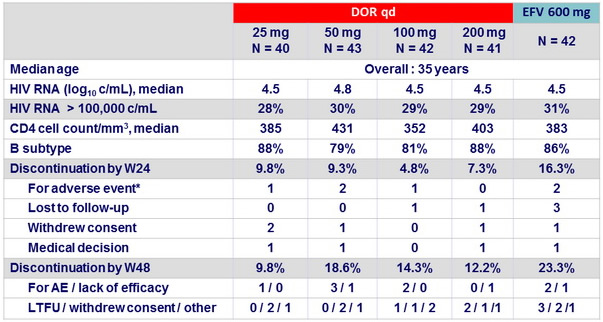
* DOR 25 mg : stupor (n = 1), DOR 50 mg: abdominal pain/nausea/insomnia (n = 1), sleep disorder (n = 1), DOR 100 mg : hallucinations
(n = 1), EFV : right-sided dysesthesia (n = 1), hallucinations (n = 1)
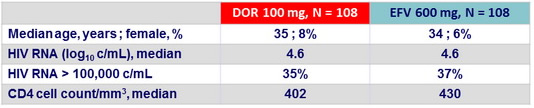
Baseline characteristics (Parts 1 and 2)
CNS events at W8, all causality
(Parts 1 and 2)
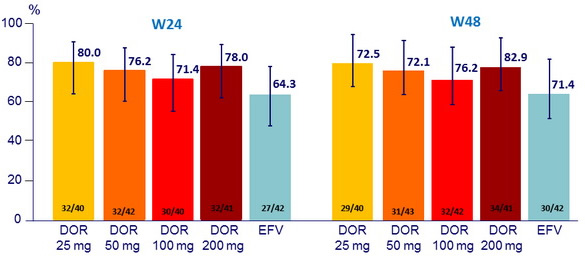
Response to treatment, HIV RNA < 40 c/ mL (ITT, NC = F)
Mean change in CD4/mm 3 at W48
- DOR all doses : + 168
- EFV : + 179
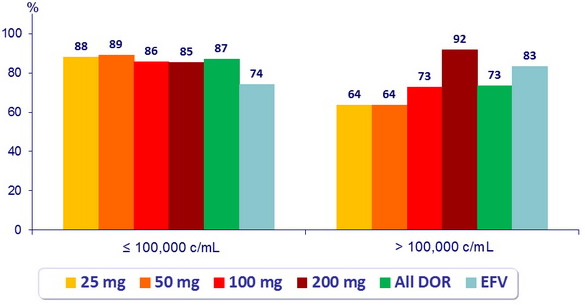
HIV RNA < 40 c/ mL (ITT, NC = F) at W48 by screening HIV RNA
Virologic failure definition
- Non-response : HIV RNA never < 40 c/mL by Week 24, or
- Rebound : after initial response of HIV RNA < 40 c/mL, 2 consecutive
HIV RNA ≥ 40 c/mL at least 1 week apart, at or after Week 24
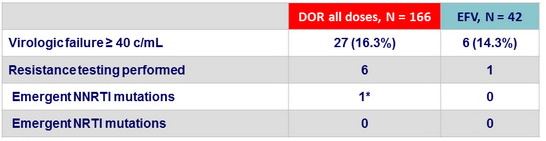
Criteria for resistance testing
Resistance data at virologic failure, W48
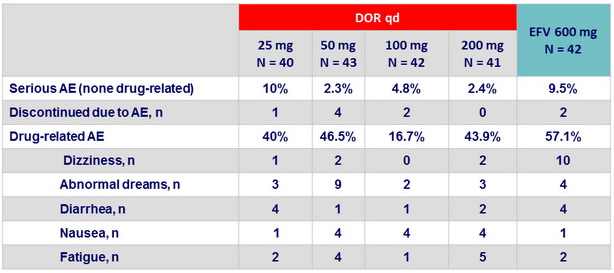
Clinical adverse events at W48 (Part 1)
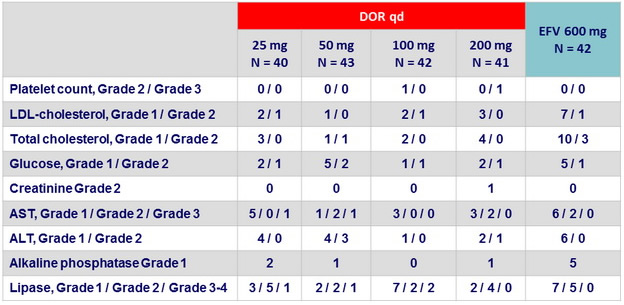
Laboratory abnormalities at W48 (Part 1 ), N
 Back to Table of Contents
Back to Table of Contents



