Lalezari J. Lancet HIV 2015; 2:e427-37 ; Thompson M. CROI 2015, Abs. 545
Type of ARV Trial
Phase 2 of new ARVs
» Fostemsavir, prodrug of temsavir (attachment inhibitor)
» FOS + RAL + TDF vs ATV/r + RAL + TDF
Phase 2 of new ARVs
» Fostemsavir, prodrug of temsavir (attachment inhibitor)
» FOS + RAL + TDF vs ATV/r + RAL + TDF
Drugs
Fostemsavir, RAL, ATV/r, TDF
Fostemsavir, RAL, ATV/r, TDF
- Virologic response rates (mITT and observed) and immunologic responses were similar across the fostemsavir and ATV/r arms through Week 48
- All fostemsavir doses were generally well tolerated with no dose-response safety signals reported
- Continuation dose of fostemsavir 1200 mg QD for the Phase IIb study
- Phase III study in heavily treatment-experienced patients with limited therapeutic options
- Phase III dose : 600 mg BID
- Subjects enrolled regardless of baseline susceptibility to temsavir
- A retrospective analysis will be conducted to determine whether a baseline phenotypic assay is necessary in the future
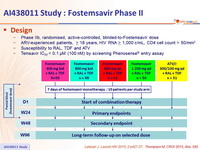
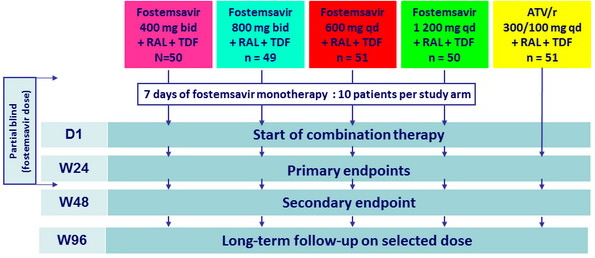
Design :
- Phase IIb, randomised, active-controlled, bl inded -to-fostemsavir dose
- ARV-experienced patients, > 18 years, HIV RNA > 1,000 c/ mL, CD4 cell count > 50/mm3
- Susceptibility to RAL, TDF and ATV
- Temsavir IC 50 < 0.1 μM (100 nM ) by screening Phenosense ® entry assay
Objective :
- Primary endpoints (W24) :
- % HIV-1 RNA < 50 c/ mL
- % of SAEs and AEs leading to discontinuation
- Secondary endpoints (W48) :
- %HIV-1 RNA < 50 c/ mL
- Change in CD4 T- cell count from baseline
- % SAEs and AEs leading to discontinuation
Baseline characteristics and patient disposition
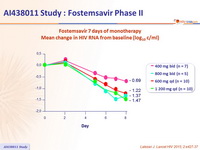
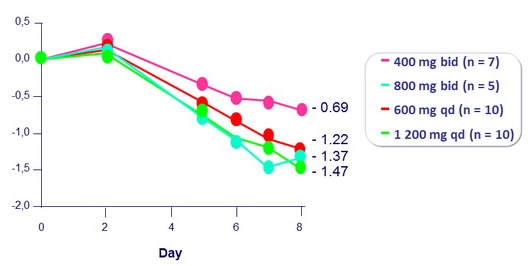
Fostemsavir 7 days of monotherapy
Mean change in HIV RNA from baseline (log 10 c/ml)
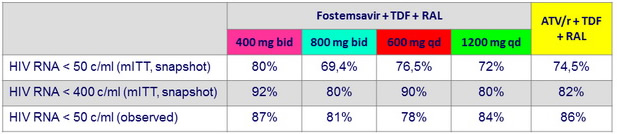
HIV RNA < 50 c/ mL or < 400 c/ mL at W24
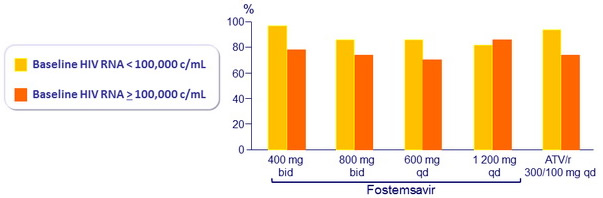
HIV RNA < 50 c/ml at W24 by baseline HIV RNA (observed )
HIV RNA < 50 c/ mL or < 400 c/ mL at W48, mITT snapshot
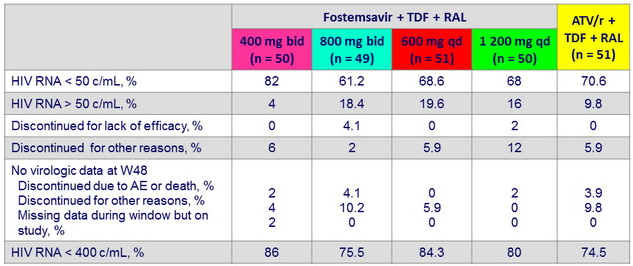
HIV RNA < 50 c/ mL at W48, observed
![]()
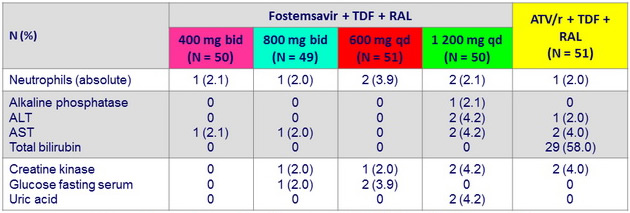
Safety data through W48
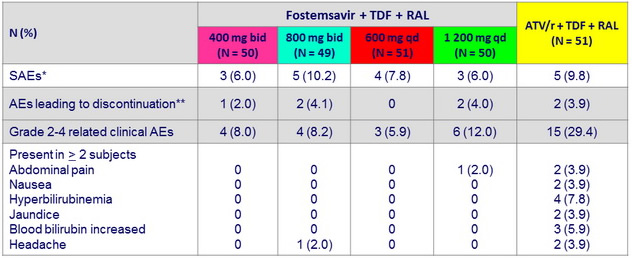
* Anal abscess, herpes encephalitis, overdose (3), extrapulmonary tuberculosis (2), herpes zoster, abdominal pain, myalgia,
spontaneous abortion, acute renal failure, cellulitis (2), lymphangitis, chronic cholecystitis, back pain, pneumonia, pyelonephritis,
diarrhea, cholelithiasis, migraine
** Illegal substance use, extrapulmonary tuberculosis (3), acute renal failure, abdominal distension, flatulence, nausea, jaundice ; 6/7 AE leading to discontinuation in first 24 weeks
Grade 3-4 laboratory abnormalities (≥ 2 subjects )
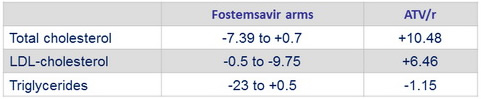
Mean change from baseline at W48 in fasting lipids, mg/ dL