Sax PE. Lancet HIV 2017; 4:e154-e160
Type of ARV Trial
Phase 2 of new ARVs
» Bictegravir
» BIC +/FTC/TAF vs DTG + FTC/TAF
Phase 2 of new ARVs
» Bictegravir
» BIC +/FTC/TAF vs DTG + FTC/TAF
Drugs
BIC/FTC/TAF, DTG, FTC/TAF, TAF, FTC
BIC/FTC/TAF, DTG, FTC/TAF, TAF, FTC
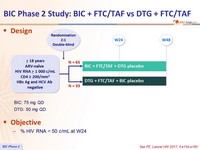
Design :
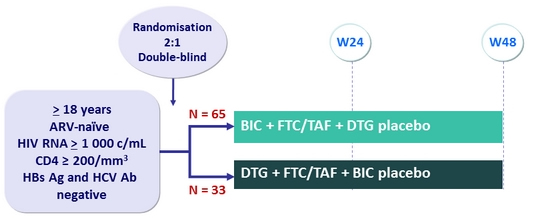
BIC: 75 mg QDµ
DTG: 50 mg QD
Objective :
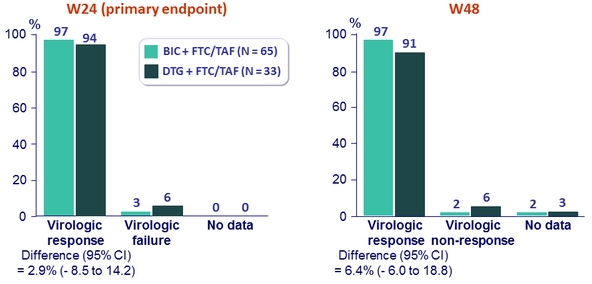
- % HIV RNA < 50 c/mL at W24
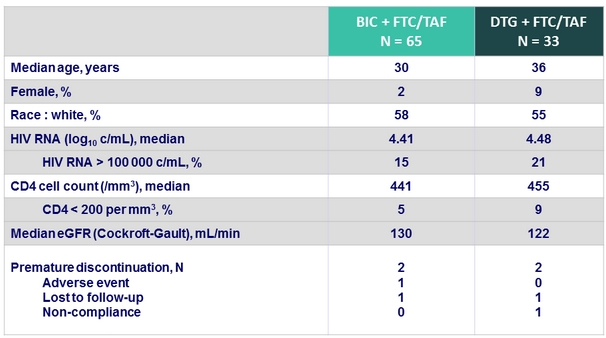
Baseline characteristics and patient disposition
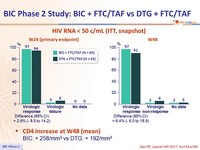
HIV RNA < 50 c/ml (ITT, snapshot )
CD4 increase at W48 (mean)
- BIC: + 258/mm3 vs DTG: + 192/mm3
Adverse events, %
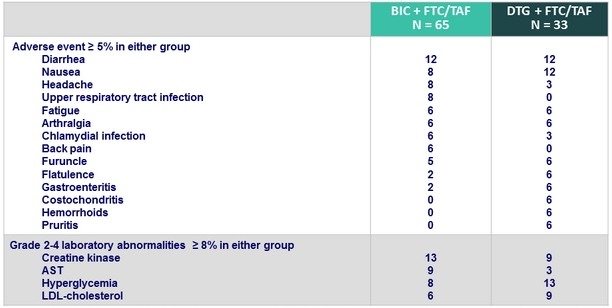
- 1 patient in the BIC + FTC/TAF group with a past history of urticaria and atopic dermatitis discontinued study drug after W24 due to urticaria