Encore1 Study Group Lancet. 2014 Apr 26;383(9927):1474-82 & Lancet Infect Dis 2015;15:793-802
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» NNRTI vs NNRTI
» EFV 600 + FTC/TDF vs EFV 400 + FTC/TDF
Head-to-head comparative trials for first line ART since 2006
» NNRTI vs NNRTI
» EFV 600 + FTC/TDF vs EFV 400 + FTC/TDF
Drugs
EFV 400, EFV 600, FTC/TDF, TDF, FTC
EFV 400, EFV 600, FTC/TDF, TDF, FTC
- A reduced dose of 400 mg EFV QD is non-inferior to the standard dose of 600 mg QD, when combined with TDF/FTC over 96 weeks in ART-naive adults with HIV-1 infection
- Overall, the frequency of adverse events did not differ and there was no evidence of difference in treatment cessation between groups
- However, adverse events related to the study drug were more frequent with 600 mg EFV than with 400 mg, and discontinuation due to adverse events more frequent with EFV 600 mg
- Quality of life, negative emotional state, and efavirenz side-effect based on specific questionnaires did not differ between EFV 600 and 400 mg QD
- These findings provide an opportunity to reduce the unit costs of treatment and care models that are based on EFV use (caution when used with rifampicin )
Design
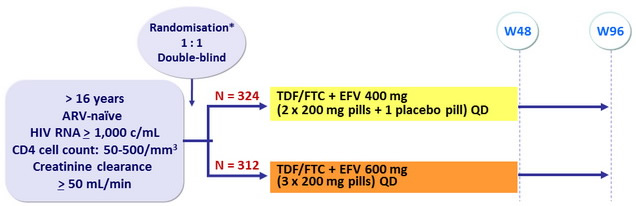
*Randomisation was stratified by clinical site and by HIV RNA (≤ or > 100,000 c/mL) at screening
Objective
- Non inferiority of EFV 400 mg at W48: % HIV RNA < 200 c/mL by modified intention to treat analysis (all randomised participants who received at least 1 dose of study drug and at least one follow-up visit), 2-sided significance level of 5%, lower margin of the 95% CI for the difference = -10%, 90% power
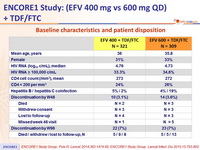
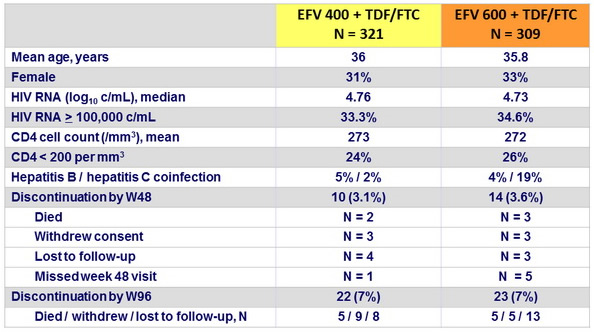
Baseline characteristics and patient disposition
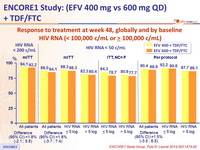
Response to treatment at week 48, globally and by baseline
HIV RNA (< 100,000 c/mL or ≥ 100,000 c/mL)
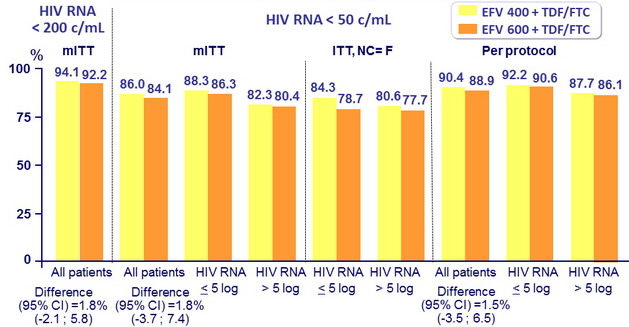
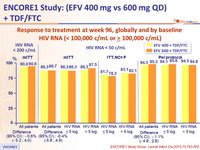
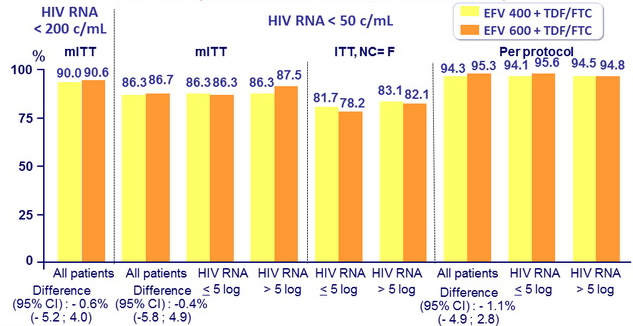
Response to treatment at week 96, globally and by baseline
HIV RNA (< 100,000 c/mL or ≥ 100,000 c/mL)
Mean CD4 cell counts increase at W96
- + 235/mm3 in the EFV 400 group
- + 209/mm3 in the EFV 600 group (p = 0.018)
No difference between groups in the mean change
- In CD4 cell percentage
- In CD8 cell counts
- Of total lymphocyte counts
At 96 weeks
- Change in DASS-21 depression, anxiety, and stress Z scores did not differ between the groups
Adverse events at week 48
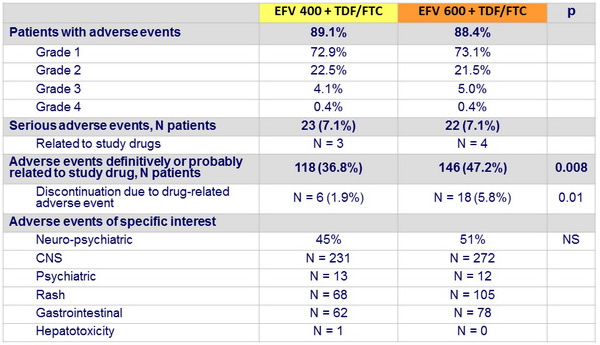
Other safety aspects at week 48
- By week 48, discontinuation of EFV : 26 (8%) in EFV 400 vs 34 (11%) in EFV 600
- Frequency of serious adverse events was similar in both groups
- No difference between randomised groups in quality of life, depression, anxiety and stress, and EFV-related symptoms over 48 weeks
- No significant differences between EFV 400 and EFV 600 in change from baseline to week 48 for most laboratory parameters, except
- Neutrophils
- Mean change in creatinine clearance : 1.29 mL/min vs – 2.17 mL/min
- Mean alkaline phosphatase increase : + 26 vs + 33 IU/L
At Week 96
- Only significant between-group difference : mean change in alkaline phosphatase : + 21 vs + 27 U/L (p = 0.046)
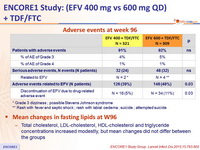
Adverse events at week 96
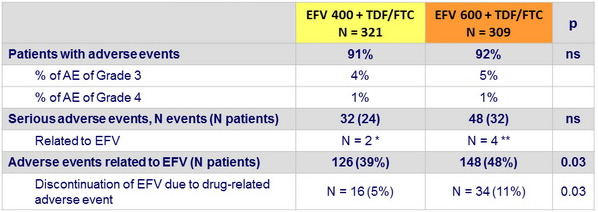
* Grade 3 dizziness ; possible Stevens Johnson syndrome
** Rash with fever and septic shock ; rash with labial oedema ; suicide ; attempted suicide
Mean changes in fasting lipids at W96
- Total cholesterol, LDL-cholesterol, HDL-cholesterol and triglyceride concentrations increased modestly, but mean changes did not differ between the groups
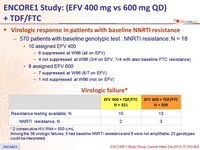
Virologic response in patients with baseline NNRTI resistance
- 570 patients with baseline genotypic test : NNRTI resistance, N = 18
- 10 assigned EFV 400
- 6 suppressed at W96 (all on EFV)
- 4 not suppressed at W96 (3/4 on EFV, 1/4 with also baseline FTC resistance)
- 8 assigned EFV 600
- 7 suppressed at W96 (6/7 on EFV)
- 1 not suppressed at W96 (not on EFV)
- 10 assigned EFV 400
Virologic failure*
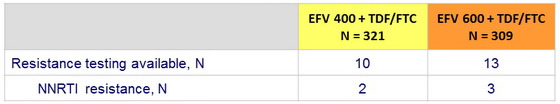
* 2 consecutive HIV RNA > 500 c/ mL
Among the 36 virologic failures, 5 had baseline NNRTI resistance and 8 were not amplifiable, 23 genotypes could be interpreted