Echeverria P. PLoS One. 2014 Feb 4;9(2):e84676
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch PI to NNRTI
» ETR + 2 NRTI
Switch studies in virologically suppressed patients
» Switch PI to NNRTI
» ETR + 2 NRTI
Drugs
ETR, 2 NRTI
ETR, 2 NRTI
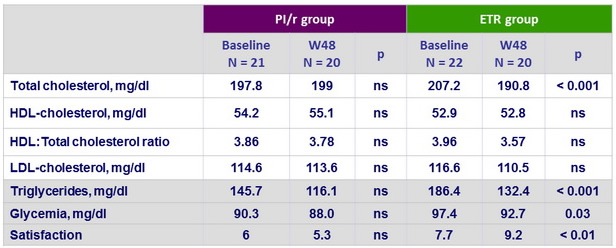
- Switch from a PI-based regimen to a once-daily combination based on ETR maintained undetectable VL during 48 weeks in virologically suppressed HIV-infected patients while lipid profile and patient satisfaction improved significantly
Design
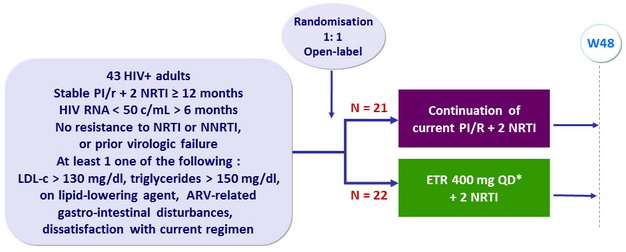
Objective
- Primary Endpoint: % HIV RNA < 50 c/mL, on-treatment and ITT, M=F
- Secondary endpoints: change in CD4, CD8, metabolic parameters (lipids and glucose), safety, patient satisfaction
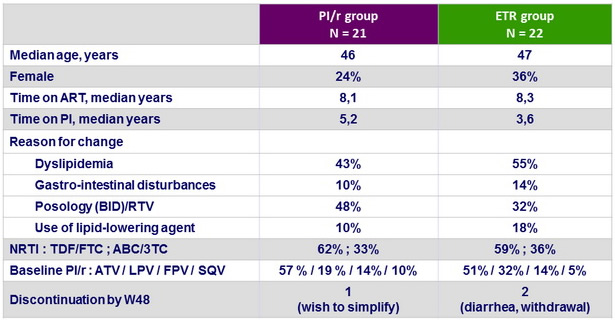
Baseline characteristics and disposition
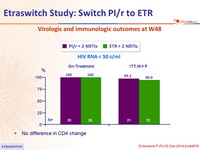
Virologic and immunologic outcomes at W48
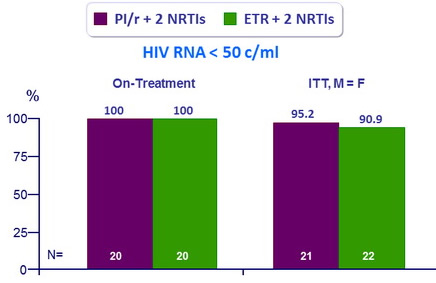
- No difference in CD4 change
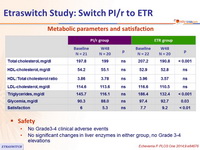
Metabolic parameters and satisfaction
Safety
- No Grade3-4 clinical adverse events
- No significant changes in liver enzymes in either group, no Grade 3-4 elevations