Orkin C. CROI 2019, Abs. 140LB; Murray M, IAS 2019, Abs. MOPEB258
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to INSTI + NNRTI
» CAB LA + RPV LA IM
Switch studies in virologically suppressed patients
» Switch to INSTI + NNRTI
» CAB LA + RPV LA IM
Drugs
CAB LA, RPV LA, ABC/3TC
CAB LA, RPV LA, ABC/3TC
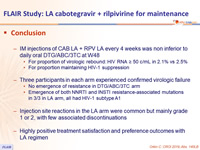
- Conclusion
- IM injections of CAB LA + RPV LA every 4 weeks was non inferior to daily oral DTG/ABC/3TC at W48
- For proportion of virologic rebound: HIV RNA ≥ 50 c/mL in 2.1% vs 2.5%
- For proportion maintaining HIV-1 suppression
- Three participants in each arm experienced confirmed virologic failure
- No emergence of resistance in DTG/ABC/3TC arm
- Emergence of both NNRTI and INSTI resistance-associated mutations in 3/3 in LA arm, all had HIV-1 subtype A1
- Injection site reactions in the LA arm were common but mainly grade 1 or 2, with few associated discontinuations
- Highly positive treatment satisfaction and preference outcomes with LA regimen
- IM injections of CAB LA + RPV LA every 4 weeks was non inferior to daily oral DTG/ABC/3TC at W48
Design
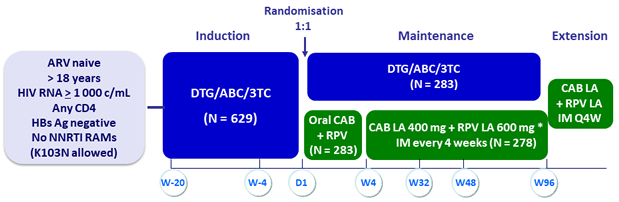
W -4: Confirm HIV RNA < 50 c/mL
* Loading dose at W4: CAB 600 mg + RPV 900 mg
- If HLA-B*5701 positive: 2 alternative non-ABC NRTI allowed
Objective
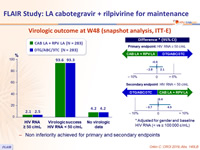
- Primary: % HIV RNA ≥ 50 c/mL at W48 of maintenance phase with monthly IM CAB LA + RPV LA (ITT, snapshot algorithm) ; non-inferiority if upper margin of a two-sided 95% CI for the difference = 6%
- Secondary: HIV RNA < 50 c/mL at W48, safety, resistance emergence, PRO, participant’s preference of the LA regimen, W96 efficacy and safety
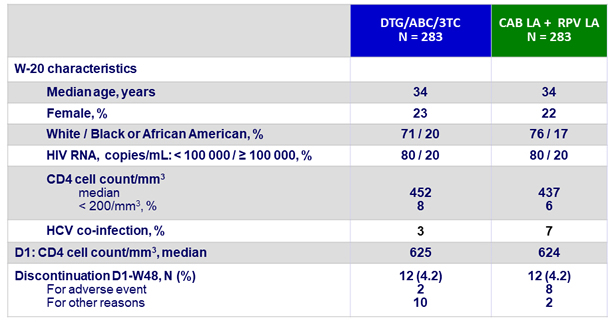
Baseline characteristics (ITT-maintenance exposed) and patient disposition
Virologic outcome at W48 (snapshot analysis, ITT-E)
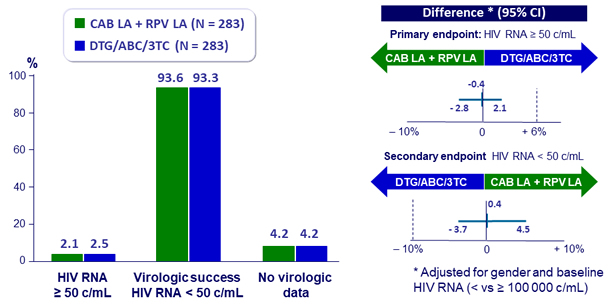
- Non inferiority achieved for primary and secondary endpoints
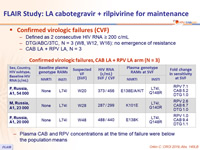
Confirmed virologic failures (CVF)
- Defined as 2 consecutive HIV RNA ≥ 200 c/mL
- DTG/ABC/3TC, N = 3 (W8, W12, W16): no emergence of resistance
- CAB LA + RPV LA, N = 3
Confirmed virologic failures, CAB LA + RPV LA arm (N = 3)
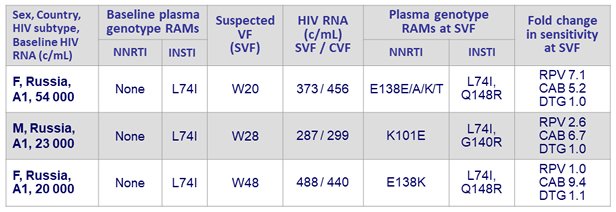
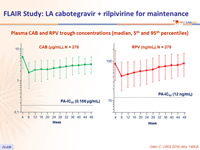
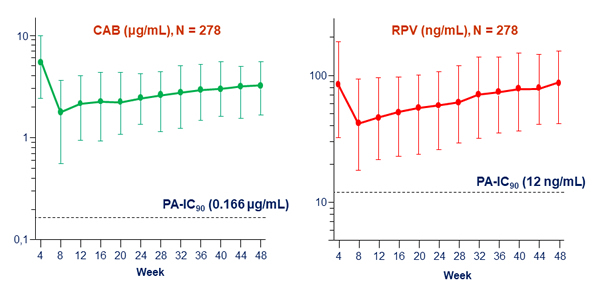
- Plasma CAB and RPV concentrations at the time of failure were below the population means
Plasma CAB and RPV trough concentrations (median, 5th and 95th percentiles)
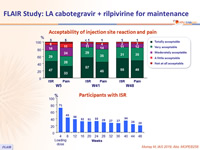
Adverse events by W48
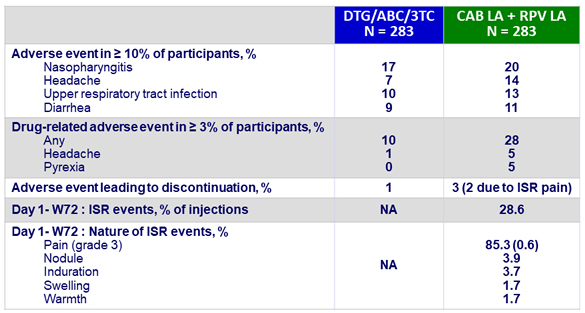
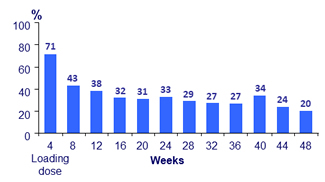
- Over time, incidence of ISR decreased (from 71% at W4 to 20% at W48)
- 99% of ISR were grade 1-2 and most (88%) resolved within ≤ 7 days (median : 3 days)
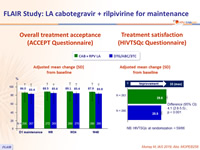
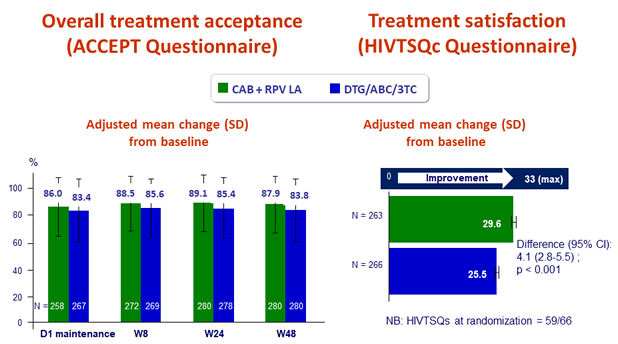
Patient reported outcomes
- Change in satisfaction with current treatment vs induction phase treatment (HIVTSQc at W48), mean total score
- LA CAB + RPV: 29.6/33
- DTG/ABC/3TC: 25.5/33 (p < 0.001)
- Participant preference at W48
- Preferred LA: 91%
- Preferred daily oral therapy: 1%
Acceptability of injection site reaction and pain
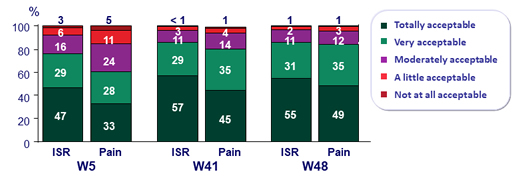
Participants with ISR