Van Lunzen J. JAIDS 2016;71:538-43 & IAC 2014, Melbourne, Abs. LBPE19
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to RAL + PI/r
» RAL + ATV/r vs ATV/r + FTC/TDF
Switch studies in virologically suppressed patients
» Switch to RAL + PI/r
» RAL + ATV/r vs ATV/r + FTC/TDF
Drugs
RAL, ATV/r, FTC/TDF, TDF, FTC
RAL, ATV/r, FTC/TDF, TDF, FTC
- In virologically suppressed patients on a triple-drug antiretroviral regimen, switching to ATV/r + RAL resulted in a lower maintenance of virologic suppression and a higher incidence of virologic rebound than in the ATV/r + TDF/FTC group at Week 24 and Week 48
- In addition, tolerability issues and treatment discontinuation occurred more frequently and adherence was lower with ATV/r + RAL
- This pilot study did not support switching to ATV/r + RAL for safety/tolerability reasons in treatment-experienced patients with virological suppression
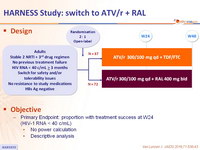
Design
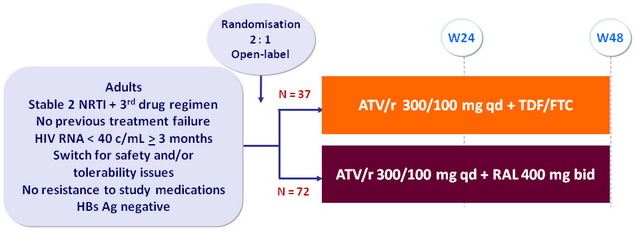
Objective
- Primary Endpoint: proportion with treatment success at W24 (HIV-1 RNA < 40 c/mL)
- No power calculation
- Descriptive analysis
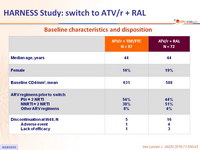
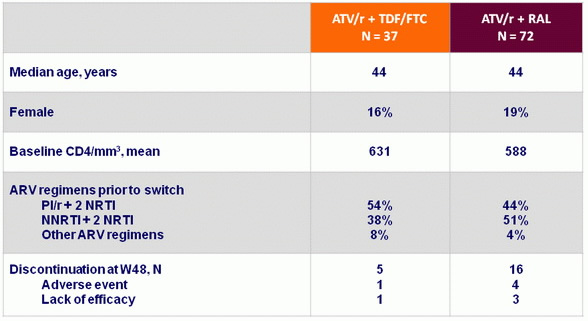
Baseline characteristics and disposition
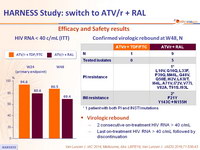
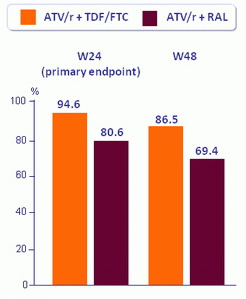
Efficacy and Safety results
HIV RNA < 40 c/mL (ITT)
Confirmed virologic rebound at W48, N
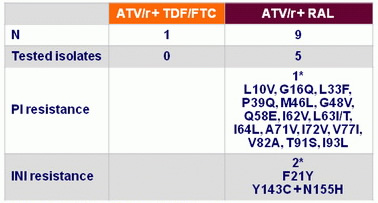
* 1 patient with both PI and INSTI mutations
Virologic rebound
- 2 consecutive on-treatment HIV RNA > 40 c/mL
- Last on-treatment HIV RNA > 40 c/mL followed by discontinuation
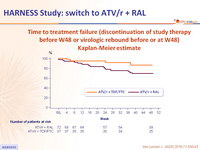
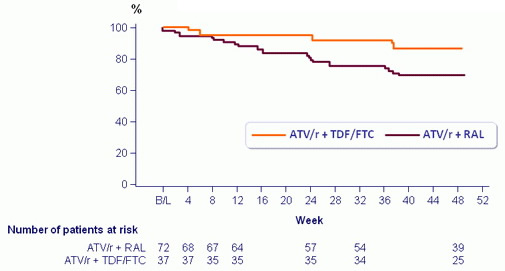
Time to treatment failure (discontinuation of study therapy before W48
or virologic rebound before or at W48) - Kaplan-Meier estimate
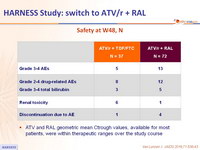
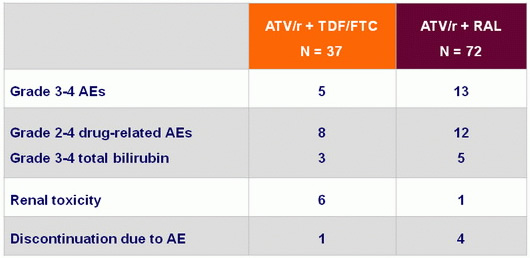
Safety at W48, N
- ATV and RAL geometric mean Ctrough values, available for most patients, were within therapeutic ranges over the study course