Maggiolo F. J Acquir Immune Defic Syndr. 2016 May 1;72(1):46-51
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to DRV/r + RPV
Switch studies in virologically suppressed patients
» Switch to DRV/r + RPV
Drugs
DRV/r, RPV, 2 NRTI
DRV/r, RPV, 2 NRTI
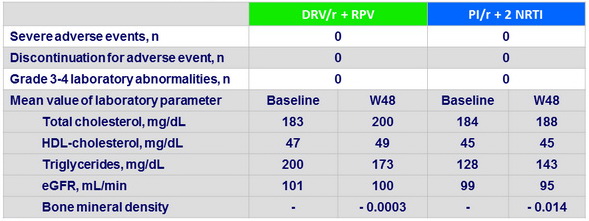
- A rilpivirine plus ritonavir-boosted DRV dual therapy was not inferior over 48 weeks to a standard boosted PI–based triple cART
- The dual therapy did not negatively affect lipid profile and renal function and was more friendly on bone mineral density
- This approach constitutes an alternative for patients experiencing nucleoside reverse transcriptase inhibitor–related toxicities
- Limitations
- Small sample size
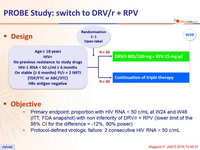
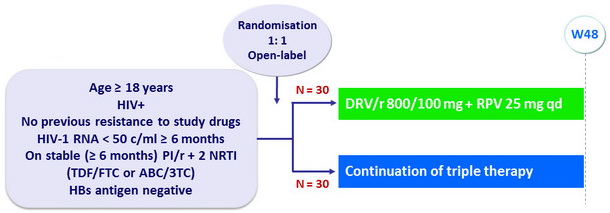
Design
Objective
- Primary endpoint: proportion with HIV RNA < 50 c/mL at W24 and W48 (ITT, FDA snapshot) with non inferiority of DRV/r + RPV (lower limit of the 95% CI for the difference = -12%, 80% power)
- Protocol-defined virologic failure: 2 consecutive HIV RNA > 50 c/mL
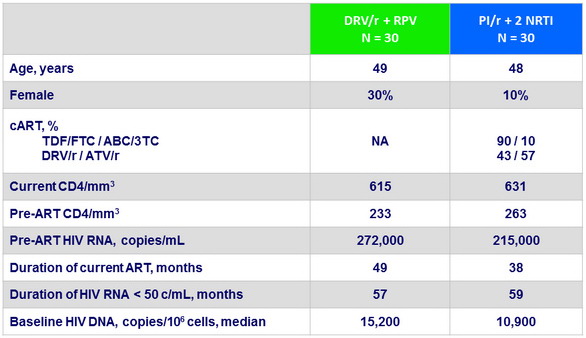
Baseline characteristics (mean)
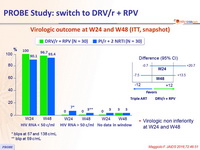
Virologic outcome at W24 and W48 (ITT, snapshot)
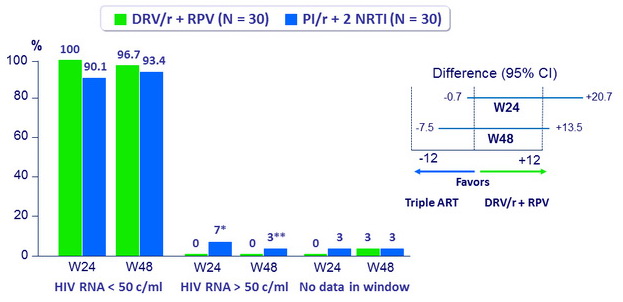
* blips at 57 and 138 c/mL
** blip at 59 c/mL
- Virologic non inferiority at W24 and W48
Secondary endpoints at W48
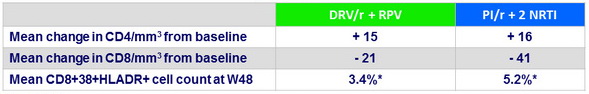
* p = 0.018
Safety and Tolerability at W48