Cohen C. AIDS 2014;28:989-97 ; Porter DP. HIV Clin Trials 2015;16:30-8 ; Van Lunzen J. AIDS 2016;30:251-9
Head-to-head comparative trials for first line ART since 2006
» NNRTI vs NNRTI
» RPV/FTC/TDF vs EFV/FTC/TDF
RPV, EFV 600, FTC/TDF, TDF, FTC
- In treatment-naive, HIV-1-infected adults, 96-week RPV/FTC/TDF treatment demonstrated noninferior efficacy and better tolerability than EFV/FTC/TDF
- Significant differences in virologic success between subgroups with HIV-1 RNA ≤ 100,000 c/mL and > 200 CD4/mmm3 could be related to the higher rate of discontinuations due to adverse events in the EFV/FTC/TDF group
- The higher virologic failure rates observed for RPV/FTC/TDF with baseline HIV-1 RNA > 500 000 c/mL and CD4+ cell count ≤ 200/mm3 were mainly due to a higher rate of discontinuation due to lack of efficacy in this group (limitation: low number of patients in those categories)
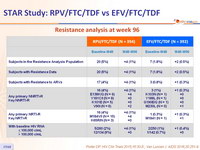
- Rates of resistance development through W96 were low (5.3% RPV/FTC/TDF ; 1.0% EFV/FTC/TDF) with infrequent emergent resistance after W48
- Development of resistance at failure: 88% RPV/FTC/TDF vs 44% EFV/FTC/TDF ; resistance in RPV/FTC/TDF group was more frequent if baseline HIV-1 RNA > 100,000 c/mL
- Better safety and tolerability profile of RPV/FTC/TDF vs. EFV/FTC/TDF over 96 weeks of treatment (limitation: open-label trial)
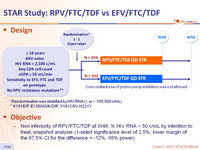
Design
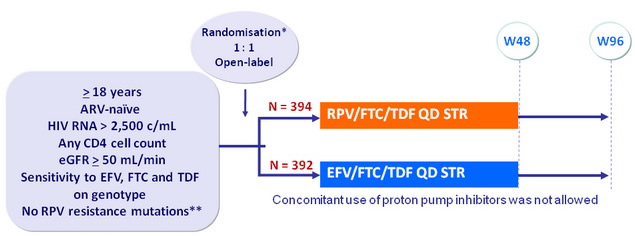
* Randomisation was stratified by HIV RNA (< or > 100,000 c/mL)
** K101E/P, E138A/G/K/Q/R, Y181C/I/V, H221Y
Objectives
- Non inferiority of RPV/FTC/TDF at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (1-sided significance level of 2.5%, lower margin of the 97.5% CI for the difference = -12%, 95% power)
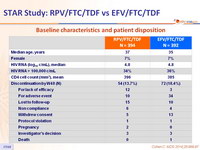
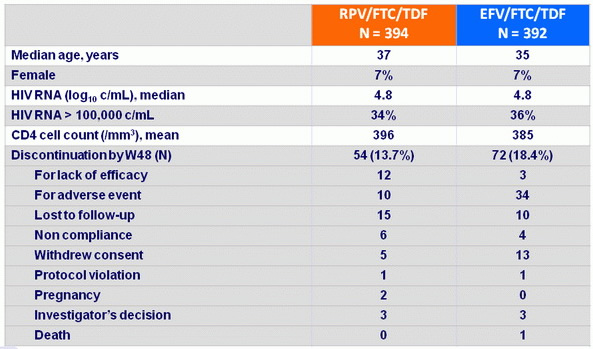
Baseline characteristics and patient disposition
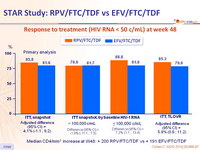
Response to treatment (HIV RNA < 50 c/mL) at week 48
Median CD4/mm3 increase at W48 : + 200 RPV/FTC/TDF vs + 191 EFV/FTC/TDF
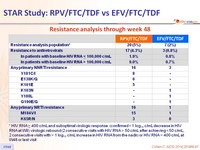
Resistance analysis through week 48
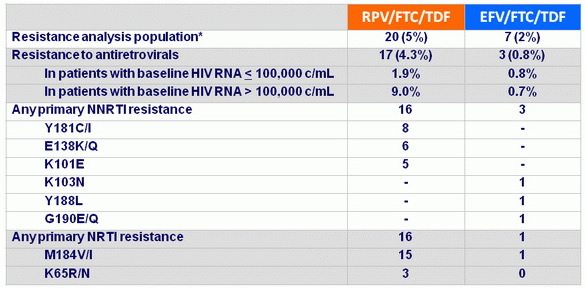
* HIV RNA ≥ 400 c/mL and suboptimal virologic response (confirmed < 1 log10 c/mL decrease in HIV RNA at W8) virologic rebound (2 consecutive visits with HIV RNA > 50 c/mL after achieving < 50 c/mL, 2 consecutive visits with > 1 log10 c/mL increase in HIV RNA from the nadir) or HIV RNA > 400 c/mL at W48 or last visit
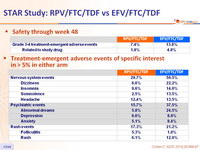
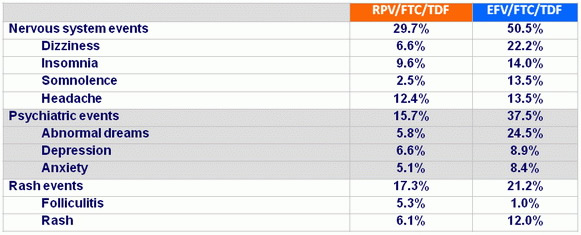
Safety through week 48
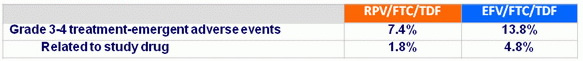
Treatment-emergent adverse events of specific interest in > 5% in either arm
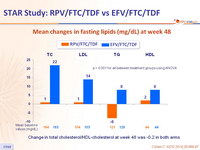
Mean changes in fasting lipids (mg/dL) at week 48
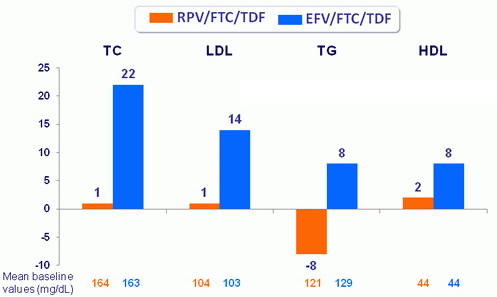
p < 0.001 for all between treatment groups using ANOVA
Change in total cholesterol/HDL-cholesterol at week 48 was -0.2 in both arms
Conclusion at week 48 :
- In treatment-naive HIV-infected patients, RPV/FTC/TDF demonstrated non inferior efficacy and improved tolerability compared with EFV/FTC/TDF, at week 48
- RPV/FTC/TDF was statistically significant superiority in efficacy for patients with baseline HIV-1 RNA ≤ 100,000 c/mL
- Virologic efficacy was similar for patients with baseline HIV-1 RNA > 100,000 c/mL
- More discontinuations due to adverse events in the EFV/FTC/TDF arm
- Significantly lower rates of nervous system and psychiatric adverse events in the RPV/FTC/TDF arm than in the EFV/FTC/TDF arm
- Differences primarily due to dizziness and abnormal dreams
- Virologic failures rates were similar between the 2 treatment arms
- A greater proportion of patients in the RPV/FTC/TDF arm developed primary emergent NRTI or NNRTI resistance mutations at virologic failure
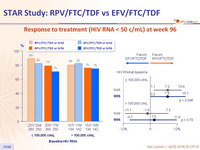
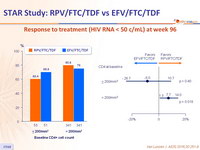
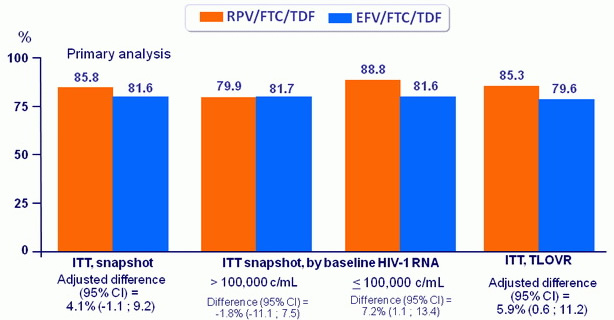
Virologic outcomes at W96, snapshot analysis
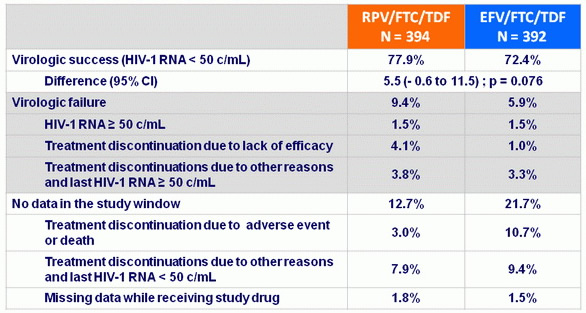
Response to treatment (HIV RNA < 50 c/mL) at week 96
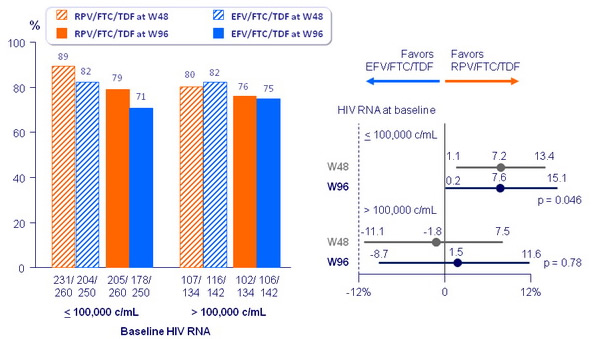
Response to treatment (HIV RNA < 50 c/mL) at week 96
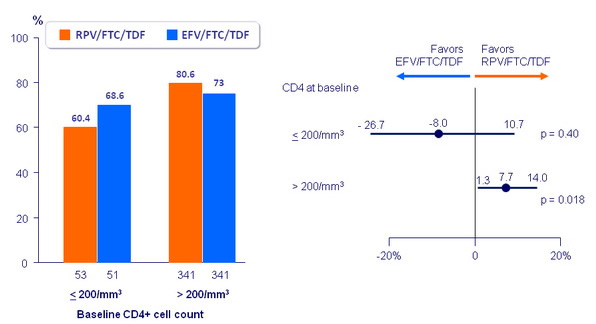
Resistance analysis at week 96
Most frequently reported treatment-emergent adverse events leading
to permanent study drug discontinuation

Grade 3–4 treatment-emergent adverse event deemed related to study drug : 2.3% RPV vs 5.6% EFV
Median changes from baseline to W96 in creatinine clearance : - 5.2 mL/min in the RPV group and + 4.3 mL/min in the EFV group
3 discontinuations for renal events : 1 in the RPV group and 2 in the EFV group
HIV Symptom Index Questionnaire at W96
- RPV/FTC/TDF: significant reduction in occurrence of 18/20 symptoms vs baseline (p ≤ 0.039)
- EFV/FTC/TDF: significant reduction in occurrence of 7/20 symptoms vs baseline (p ≤ 0.033)
- Significant between-group differences in symptom occurrence vs baseline for 8 symptoms, all favoring RPV/FTC/TDF
Overall satisfaction (HIV Treatment Satisfaction Questionnaire) at W96
- High in both groups
Quality of life (SF-12V2)
- The between-group difference in the median change from baseline at W96 for the physical health composite score was not significant
- The difference for the mental health composite score was significant, favoring RPV/FTC/TDF (p = 0.014)