Squires K. IAS 2015 Vancouver, Abs. MOLBPE08 ; Lancet HIV 2016; 3(9):e410-e420
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» INSTI vs PI
» EVG/C/FTC/TDF QD vs ATV/r + FTC/TDF
Head-to-head comparative trials for first line ART since 2006
» INSTI vs PI
» EVG/C/FTC/TDF QD vs ATV/r + FTC/TDF
Drugs
E/C/F/TDF, EVG/c, ATV/r, FTC/TDF, TDF, FTC
E/C/F/TDF, EVG/c, ATV/r, FTC/TDF, TDF, FTC
- EVG/C/FTC/TDF QD was virologically non inferior and superior to ATV + r + FTC/TDF
- Similar virologic response of the 2 regimens in different subgroups of patients, including those with high HIV RNA or CD4 < 350/mm3 at enrolment
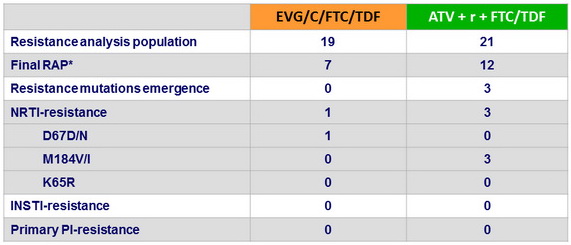
- Development of major resistance mutations occurred in
- No patients on EVG/C/FTC/TDF
- 3 patients on ATV + r + /FTC/TDF: NRTI mutations, no PI mutations
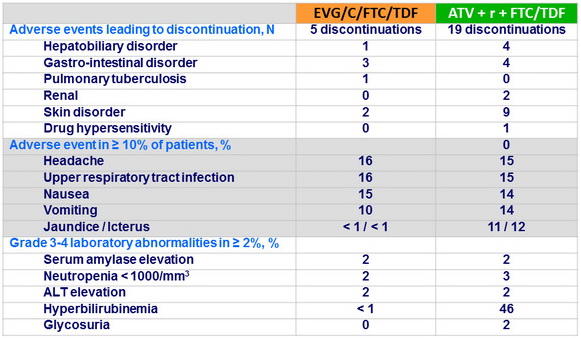
- Discontinuation because of adverse events was lower with EVG/C/FTC/TDF
- Less incidence of icterus and hyperbilirubinemia with EVG/C/FTC/TDF
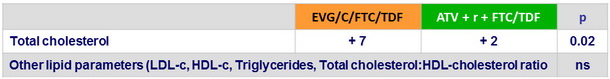
- Comparable changes in fasting lipids in both groups, except for total cholesterol which elevation was higher with EVG/C/FTC/TDF
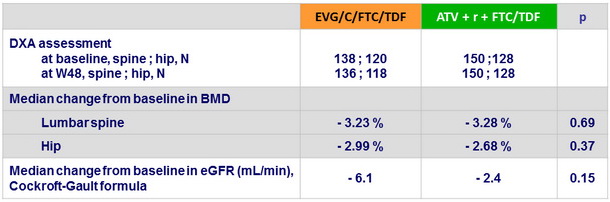
- Median decreases in estimated glomerular filtration rate and in spine and hip BMD were modest and not different between the 2 groups
Design
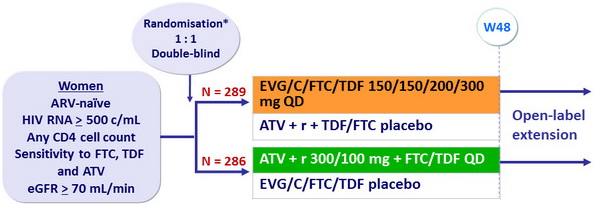
*
Randomisation was stratified by HIV RNA ( ≤ 100,000 or 100,000-400,000 or > 400,000 c/ mL )
at screening and race (black or non-black)
Objective
- Non inferiority of EVG/C/FTC/TDF at W48: % HIV RNA < 50 c/ mL by intention to treat, snapshot analysis (lower margin of the 2-sided 95% CI for the difference = -12%)
Baseline characteristics and patient disposition
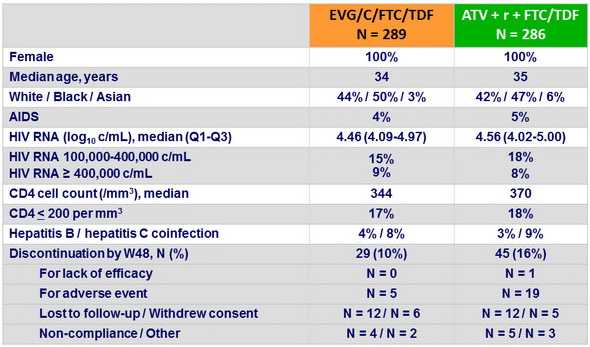
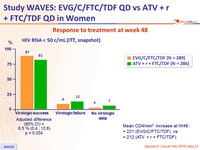
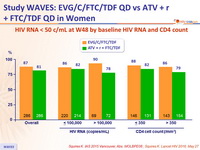
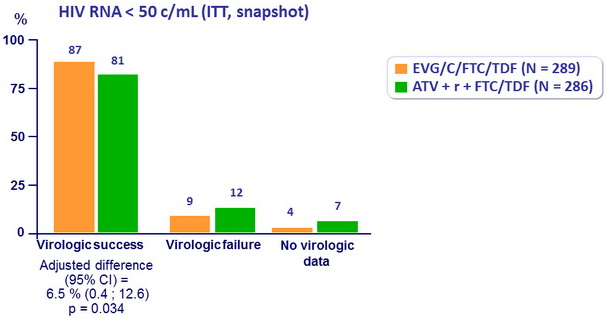
Response to treatment at week 48
Mean CD4/mm3 increase at W48 :
- + 221 (EVG/C/FTC/TDF) vs
- + 212 (ATV + r + FTC/TDF)
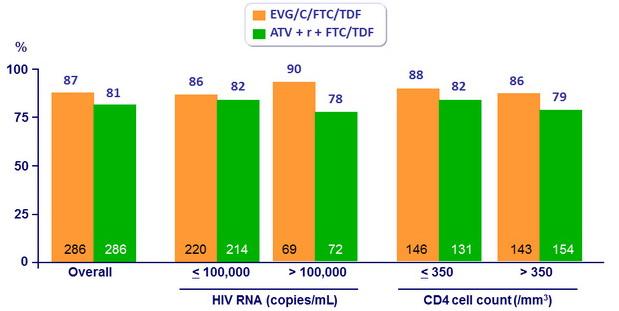
HIV RNA < 50 c/ mL at W48 by baseline HIV RNA and CD4 count
Emergence of resistance
* Criteria :
- Suboptimal response (HIV RNA ≥ 50 c/ mL and < 1 log10 reduction from baseline by W8, confirmed)
- Virologic rebound (> 400 c/ mL after achieving HIV RNA < 50 c/ mL, or 2 consecutive visits with
> 1 log10 increase from nadir)
- HIV RNA > 400 c/ mL at W48
Exclusion of patients with HIV RNA < 50 c/ mL at subsequent visits
Renal and bone mineral density (DXA) assessments
Median change from baseline in fasting lipids (mg/ dL )
Adverse events and Grade 3-4 laboratory abnormalities