Comparison of RTV vs Cobi
Study GS-US-216-0114: ATV + ritonavir + FTC/TDF QD vs ATV + cobicistat + FTC/TDF
Original article : J Infect Dis. 2013 Jul;208(1):32-9 - JE Gallant & J Acquir Immune Defic Syndr . 2015 Jul 1; 69 (3): 338-40
Last update :
23/11/2015
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- COBI was non inferior to RTV in combination with ATV plus FTC/TDF up to week 144
- Both regimens achieved high rates of virologic success
- Safety and tolerability profiles of the 2 regimens were comparable
- Once-daily COBI is a safe and effective pharmaco -enhancer of the protease inhibitor ATV
- Renal safety was comparable between treatment arms
- Discontinuation due to renal events was 2.9% in the COBI group and 3.2% in the RTV group at W144
- Proximal renal tubulopathy occurred in 7 vs 7 patients (2.0%)
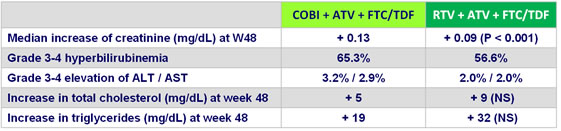
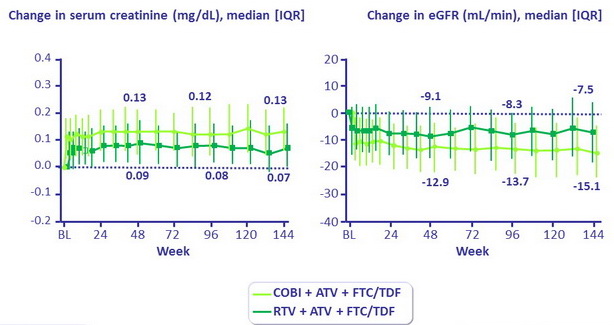
- A small, but significantly higher with COBI , increase in creatinine was seen in both groups, as early as week 2, with peak at week 8, and stabilization through 144 weeks
Design
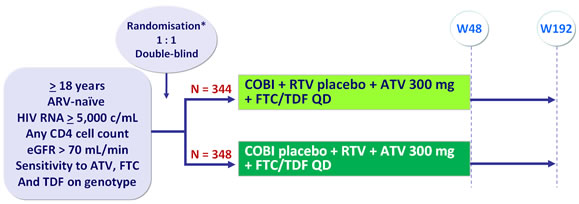
* Randomisation was stratified by HIV RNA (≤ or > 100,000 c/mL) at screening
Objective :
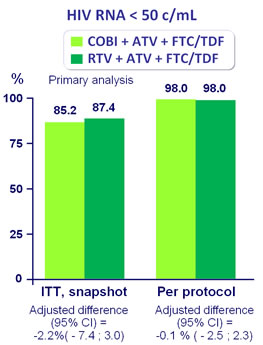
- Non inferiority of COBI compared with RTV at W48: % HIV RNA < 50 c/mL �by intention to treat, snapshot analysis (lower limit for the 95% CI for the difference = -12%, 95% power)
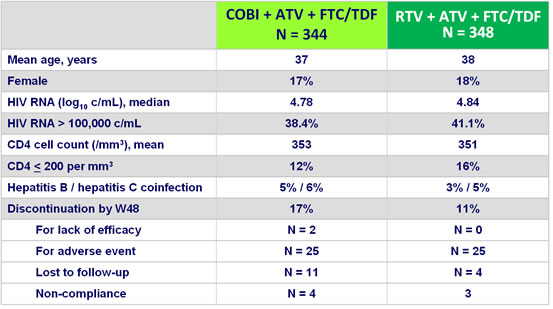
Baseline characteristics and patient disposition
Response to treatment at week 48
Viral suppression was high in both treatment arms, for various subgroups, including patients with �HIV RNA > 100,000 c/mL at baseline
Mean CD4/mm3 increase at W48 :
+ 213 COBI vs + 219 RTV
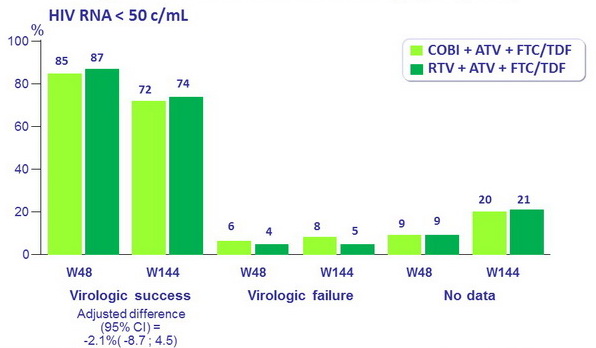
Response to treatment at
week 144 (ITT, snapshot)
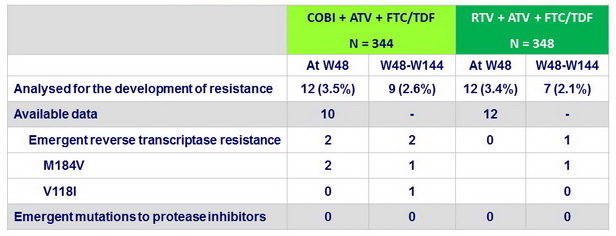
Criteria for resistance testing : confirmed HIV-1 RNA load rebound
of ≥ 400 c/mL or not obtaining HIV RNA < 400 c/mL by or after week 8
Resistance data up to week 144
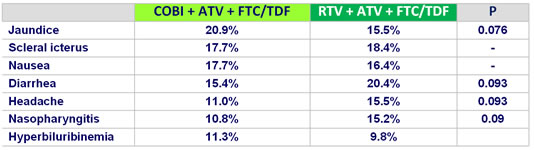
Adverse events occurring in > 10% of patients in either group (W48)
Laboratory abnormalities at W48
Adverse events leading to discontinuation of study drug
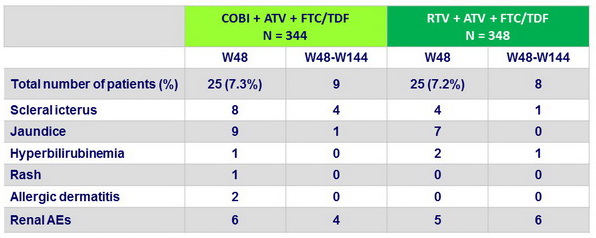
Proximal renal tubulopathy
- 7 in each group
- In 5 of the 7 patients in the COBI group and 6 of the 7 patients in the RTV group, PRT occurred after week 48
Serum Creatinine and eGFR
Median change in fasting lipids at week 144 (mg/dL)
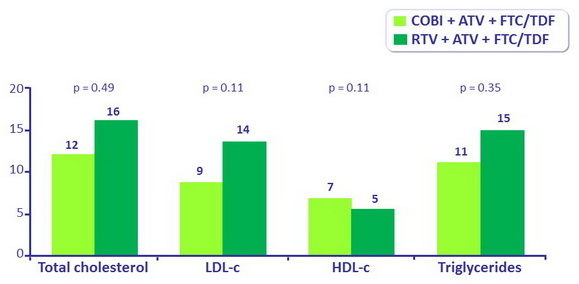
- No difference in TC:HDL ratio changes between arms (-0.3 vs -0.2)
 Back to Table of Contents
Back to Table of Contents



