Soriano V. Antivir Ther. 2011;16(3):339-48 ; Podzamczer D. HIV Med. 2011 Jul;12(6):374-82
Head-to-head comparative trials for first line ART since 2006
» NNRTI vs PI/r
» NVP + FTC/TDF vs ATV/r + FTC/TDF
ATV/r, NVP, FTC/TDF
- NVP demonstrated at week 48 non-inferior antiviral efficacy compared with ATV/r when given along with TDF/FTC, despite more drug-related discontinuations with NVP than ATV/r
- NVP BID and QD had similar efficacy and tolerability
- The application of the recommended CD4+ T-cell thresholds when initiating first-line NVP therapy probably explain relative low rate of liver enzymes increases and discontinuations for liver toxicity
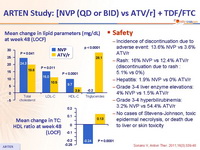
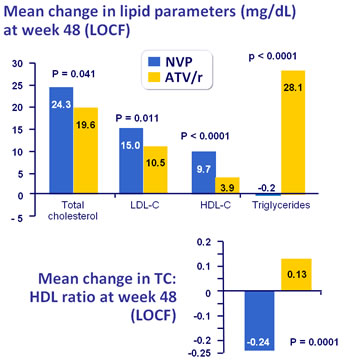
- NVP was associated with a lower atherogenic lipid profile than ATV/r
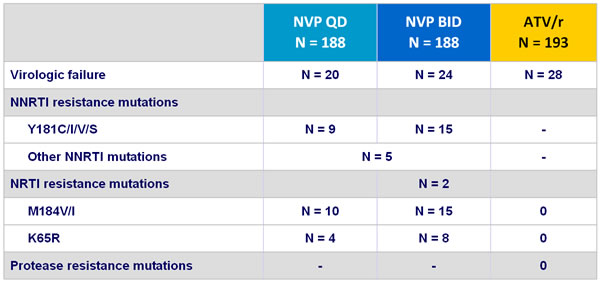
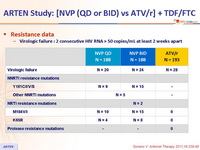
- At virologic failure, there was a high rate of resistance mutations selected by NVP and none with ATV/r
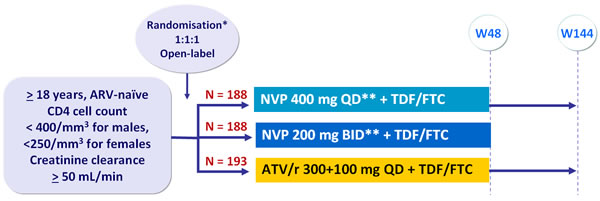
Design :
* Randomisation stratified by HIV RNA (< or > 100,000 c/mL) and CD4 (> or < 50/mm3) at screening
** Lead-in of NVP 200 mg QD for the first 2 weeks
Objective :
- Non inferiority of NVP (combined groups) compared to ATV/r for primary endpoint: % HIV RNA < 50 c/mL at W24, W36 and W48 by intention to treat with non completers equals failures, (2-sided significance level of 5%, lower margin of the 95% CI for the difference = -12%, 80% power)
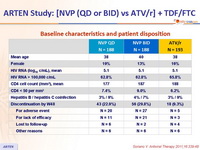
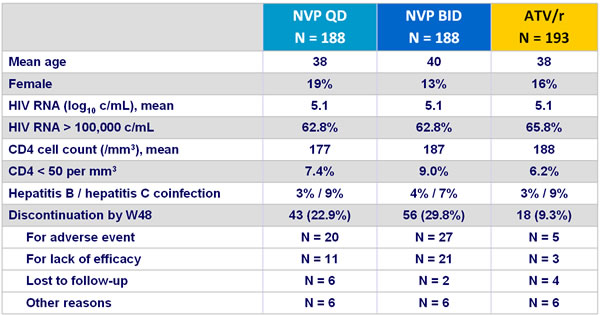
Baseline characteristics and patient disposition :
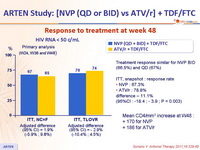
Response to treatment at week 48 :
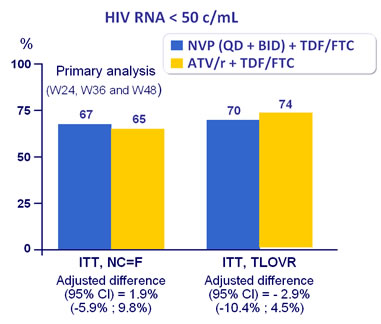 Treatment response similar for NVP BID (66.5%) and QD (67%)
Treatment response similar for NVP BID (66.5%) and QD (67%)
ITT, snapshot : response rate
-
NVP : 67.3%
-
ATV/r : 78.8%
difference – 11.1%
(95%CI : -18.4 ; - 3.9 ; P = 0.003)
Mean CD4/mm3 increase at W48 :
+ 170 for NVP
+ 186 for ATV/r
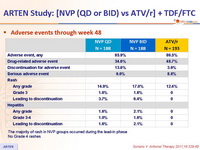
Adverse events through week 48 :
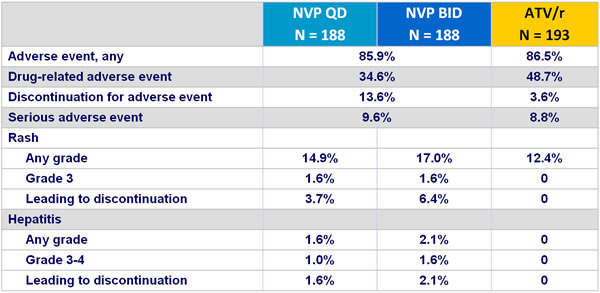
The majority of rash in NVP groups occurred during the lead-in phase
No Grade 4 rashes
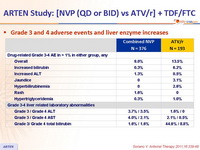
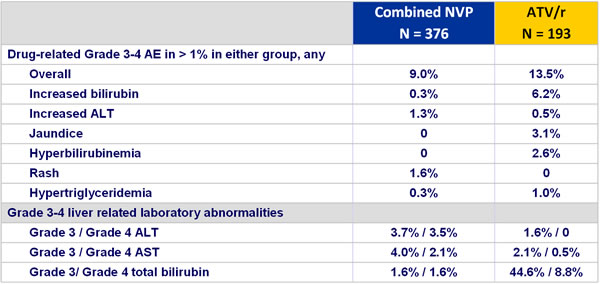
Grade 3 and 4 adverse events and liver enzyme increases :
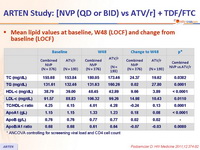
Mean lipid values at baseline, W48 (LOCF) and change from baseline (LOCF) :
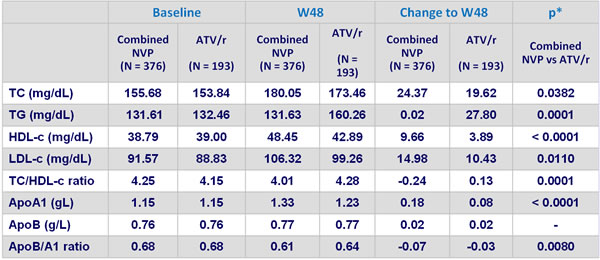
* ANCOVA controlling for screeening viral load and CD4 cell count
 Safety :
Safety :
- Incidence of discontinuation due to adverse event: 13.6% NVP vs 3.6% ATV/r
- Rash: 16% NVP vs 12.4% ATV/r (discontinuation due to rash : 5.1% vs 0%)
- Hepatitis: 1.9% NVP vs 0% ATV/r
- Grade 3-4 liver enzyme elevations: 4% NVP vs 1.5% ATV/r
- Grade 3-4 hyperbilirubinemia: 3.2% NVP vs 54.4% ATV/r
- No cases of Stevens-Johnson, toxic epidermal necrolysis, or death due to liver or skin toxicity
Resistance data :
Virologic failure : 2 consecutive HIV RNA > 50 copies/mL at least 2 weeks apart