Vernazza P. AIDS. 2007 Jun 19;21(10):1309-15
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
Drugs
ATV/r
ATV/r
- Limited pilot study, no control arm
- Caution: risk of compartmentalisation of HIV RNA replication in the CSF
Design :
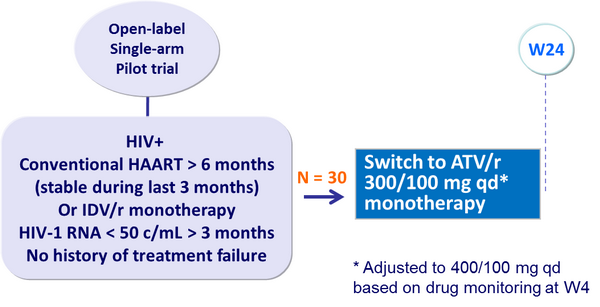
Primary endpoint :
- Confirmed virologic failure by W24 (2 consecutive HIV-1 RNA > 400 c/mL, or 3 consecutive HIV-1 RNA > 200 c/mL, or 4 consecutive HIV-1 RNA > 100 c/mL)
Other endpoints
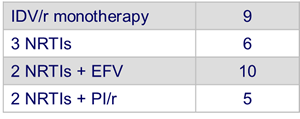
- Prior ARV therapy
- Mean CD4 cell count at inclusion = 618/mm3
- 2 virologic failures (7%)
- Among 20 patients with plasma HIV-RNA < 50 c/mL at W24, CSF HIV-1 RNA was > 100 c/mL in 3