Swindells S. CROI 2019, Abs. 139; Murray M, IAS 2019, Abs. MOAB0103
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to INSTI + NNRTI
» CAB LA + RPV LA IM
Switch studies in virologically suppressed patients
» Switch to INSTI + NNRTI
» CAB LA + RPV LA IM
Drugs
CAB LA, RPV LA, ABC/3TC
CAB LA, RPV LA, ABC/3TC
- Conclusion
- IM injections of CAB LA + RPV LA every 4 weeks was non inferior to daily oral ART at W48
- For proportion of virologic rebound: HIV RNA ≥ 50 c/mL in 1.6% vs 1.0%
- For proportion maintaining HIV-1 suppression
- Low rate (1%) of virologic failure in each arm
- No emergence of resistance in cART arm
- 2 of 3 participants on LA arm had NNRTI RAMs in baseline PBMCs, emergence of major INSTI resistance in 1/3
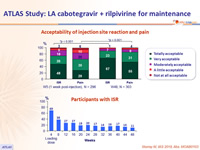
- Injection site reactions in the LA arm were common but mainly grade 1 or 2, with few associated discontinuations
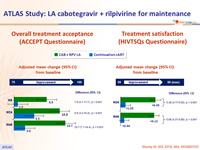
- Significant greater increase in treatment satisfaction with LA regimen
- IM injections of CAB LA + RPV LA every 4 weeks was non inferior to daily oral ART at W48
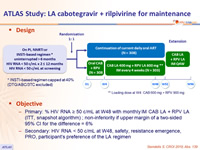
Design
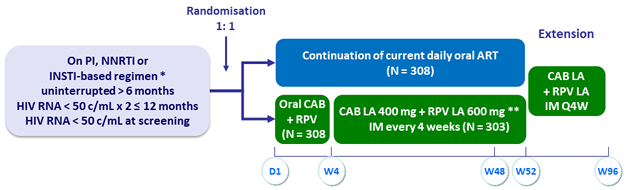
* INSTI-based regimen capped at 40% (DTG/ABC/3TC excluded)
** Loading dose at W4: CAB 600 mg + RPV 900 mg
Objectives
- Primary: % HIV RNA ≥ 50 c/mL at W48 with monthly IM CAB LA + RPV LA (ITT, snapshot algorithm) ; non-inferiority if upper margin of a two-sided 95% CI for the difference = 6%
- Secondary: HIV RNA < 50 c/mL at W48, safety, resistance emergence, PRO, participant’s preference of the LA regimen
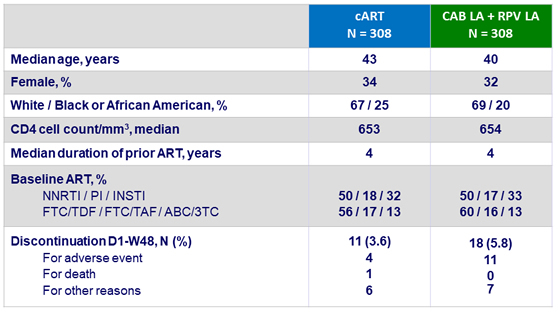
Baseline characteristics (ITT-exposed) and patient disposition
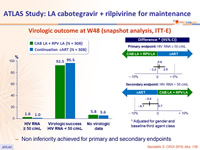
Virologic outcome at W48 (snapshot analysis, ITT-E)
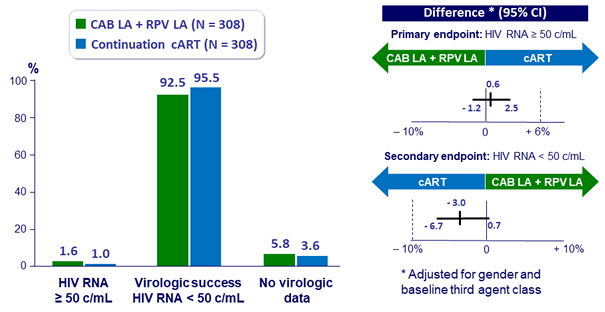
- Non inferiority achieved for primary and secondary endpoints
Confirmed virologic failures (CVF)
- Defined as 2 consecutive HIV RNA ≥ 200 c/mL
- DTG/ABC/3TC, N = 4 (W20, W20, W32, W40): no emergence of resistance
- CAB LA + RPV LA, N = 3
Confirmed virologic failures, CAB LA + RPV LA arm (N = 3)
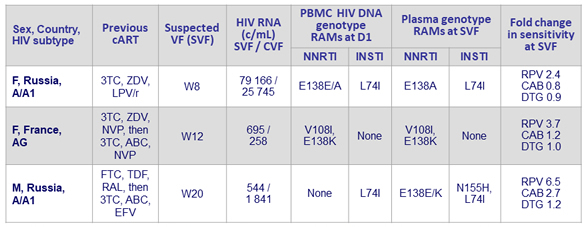
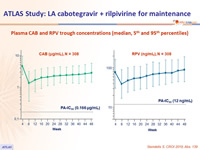
- Plasma CAB and RPV concentrations at the time of failure were below the population means
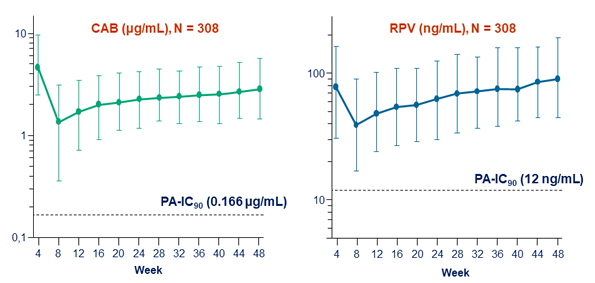
Plasma CAB and RPV trough concentrations (median, 5th and 95th percentiles)
Adverse events by W48
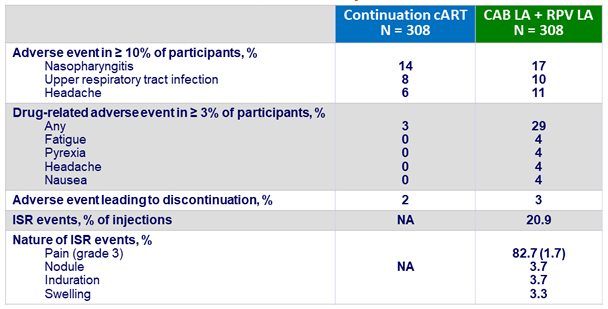
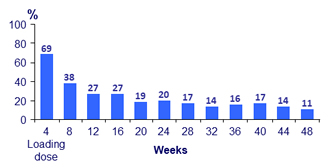
- Over time, incidence of ISR decreased (from 69% at W4 to 11% at W48)
- 99% of ISR were grade 1-2 and most (88%) resolved within ≤ 7 days (median : 3 days)
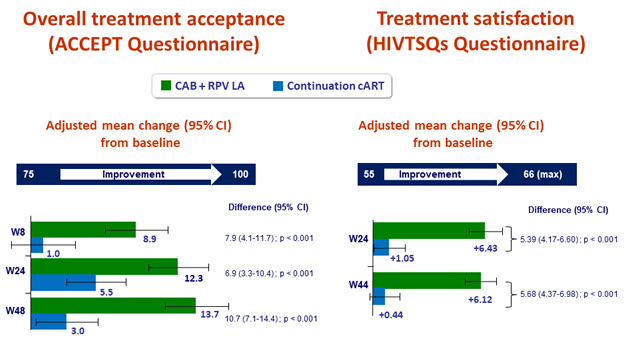
Patient reported outcomes
- Change in satisfaction with current treatment (HIVTSQs), adjusted mean change from baseline at W44
- LA CAB + RPV: + 6.12
- Continuation cART: + 1.05 (p < 0.001)
- Participant preference at W48
- Preferred LA: 86%
- Preferred daily oral therapy: 2%
Acceptability of injection site reaction and pain
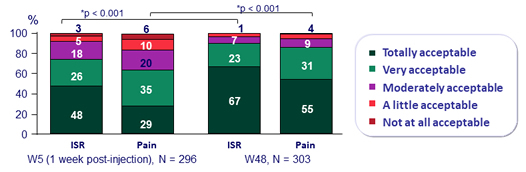
Participants with ISR