Orkin C. Clin Infect Dis. 2019 ;68:535-44
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» NNRTI vs NNRTI
» EFV/FTC/TDF vs DOR/3TC/TDF
Head-to-head comparative trials for first line ART since 2006
» NNRTI vs NNRTI
» EFV/FTC/TDF vs DOR/3TC/TDF
Drugs
DOR, EFV 600, FTC/TDF, TDF, 3TC
DOR, EFV 600, FTC/TDF, TDF, 3TC
- Summary at week 96
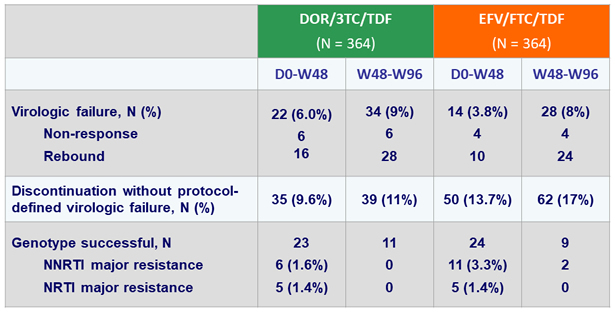
- DOR/3TC/TDF continued to be non-inferior to EFV/FTC/TDF, with no additional emergence of resistance to DOR between W48 and W96
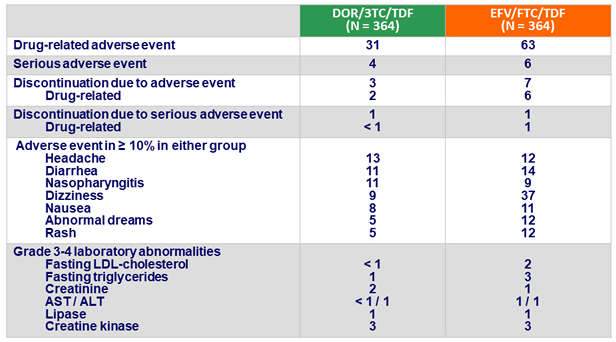
- Rate of discontinuation for adverse event was lower with DOR/3TC/TDF (3% vs 7%)
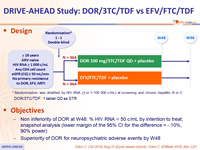
Design :
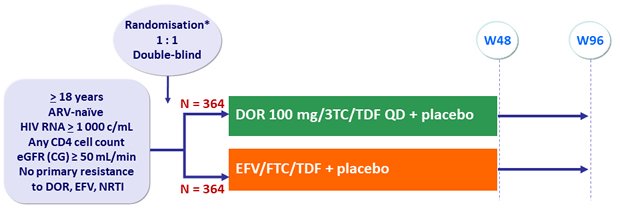
* Randomisation was stratified by HIV RNA (< or > 100 000 c/mL) at screening and chronic hepatitis B or C
-
DOR/3TC/TDF : 1 tablet qd as STR
Objectives :
- Non inferiority of DOR at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (lower margin of the 95% CI for the difference = - 10%, 90% power)
- Superiority of DOR for neuropsychiatric adverse events by W48
Baseline characteristics and patient disposition :
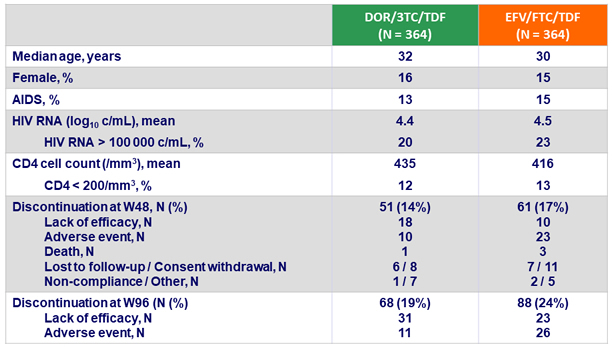
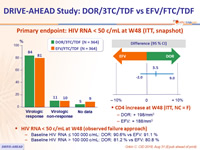
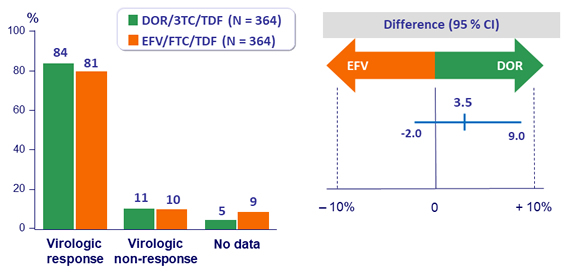
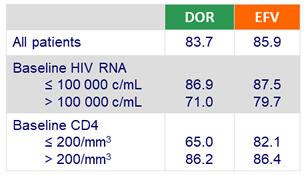
Primary endpoint: HIV RNA < 50 c/mL at W48 (ITT, snapshot)
- HIV RNA < 50 c/mL at W48 (observed failure approach)
- Baseline HIV RNA ≤ 100 000 c/mL: DOR: 90.6% vs EFV: 91.1 %
- Baseline HIV RNA > 100 000 c/mL: DOR: 81.2 % vs EFV: 80.8 %
- CD4 increase at W48 (ITT, NC = F)
- DOR: + 198/mm3
- EFV: + 188/mm3
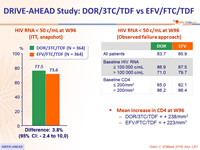
HIV RNA < 50 c/mL at W96 (ITT, snapshot)
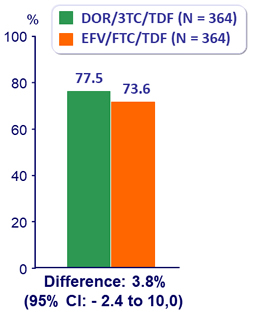
HIV RNA < 50 c/mL at W96 (Observed failure approach)
- Mean increase in CD4 at W96
- DOR/3TC/TDF = + 238/mm3
- EFV/FTC/TDF = + 223/mm3
Protocol-defined virologic failures (PDVF) at W48
- Definition
- Non response: HIV RNA ≥ 200 c/mL at W24 or W36 or confirmed HIV RNA ≥ 50 c/mL at W48
- Rebound: confirmed HIV RNA ≥ 50 c/mL after obtaining HIV RNA < 50 c/mL
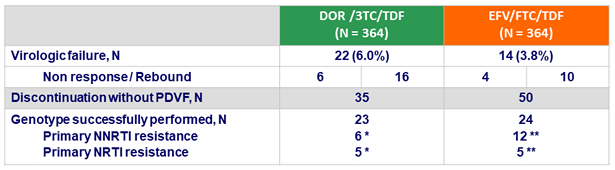
* NNRTI mutations : Y188L; V106I, F227C; V106V/I, H221H/Y, F227C; F227C; V106A, P225H, Y318Y/F; V106M/T, F227C/R ; NRTI mutations : M41L, M184V; M184V; M184V; K65R; K65K/R, M184V
** NNRTI mutations : K103N; K103N, E138E/G; K103N; G190E; K103N; K103N, M230L; G190E; K103N, V108V/I, T369T/A/I/V; K103N; K103N; K101K/N, K103N, P225P/H ; NRTI mutations : V118I, M184V; M184V; M184V; M184V, K219K/E; K65K/R, M184M/I
Virologic failure and resistance, W96
Adverse events at W48, %
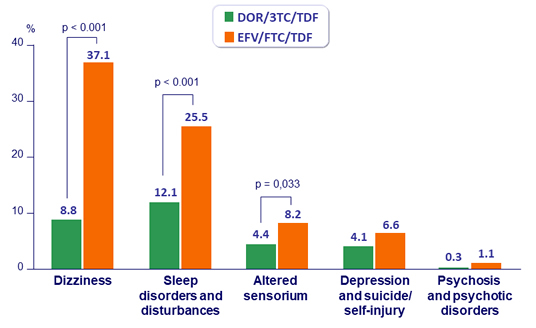
% with Predefined Neuropsychiatric Adverse Events at W48
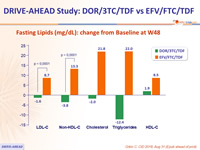
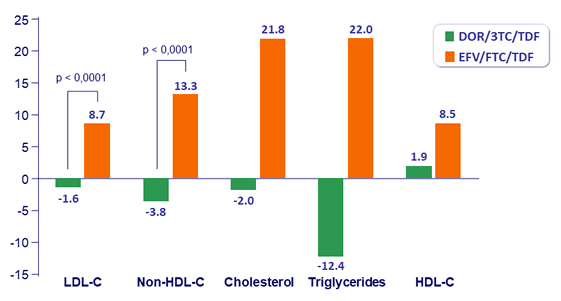
Fasting Lipids (mg/dL): change from Baseline at W48
Summary at week 48
- In treatment-naïve adults with HIV-1 infection, DOR/3TC/TDF administered once daily demonstrated:
- Antiviral potency with non-inferior efficacy to EFV/FTC/TDF regardless of baseline HIV-1 RNA
- Low rate of resistance, with only 1.6% of participants developing resistance to any study drug through W48
- DOR/3TC/TDF was generally well tolerated and safe:
- Neuropsychiatric profile superior to EFV/FTC/TDF, as measured by lower proportion of participants with neuropsychiatric adverse events in categories of dizziness, sleep disorders and disturbances, and altered sensorium
- Lipid profile superior to EFV/FTC/TDF, as assessed by difference from baseline in fasting LDL-C and non-HDL-C