Braun DL, Clin Infect Dis 2019, Janv 2, Epub ahead of print
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG monotherapy
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG monotherapy
- In patients who initiated cART < 180 days after the estimated day of a document primary HIV-1 infection and had HIV-1 RNA < 50 c/mL for more than 48 weeks, DTG monotherapy was non-inferior to cART
Design
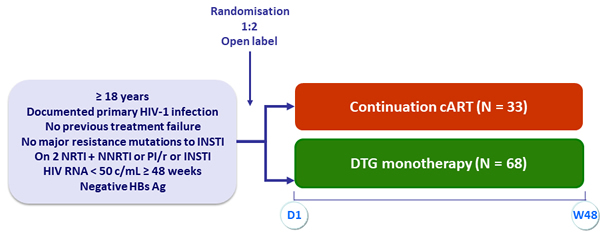
Objective
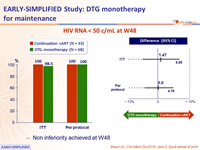
- Primary: % HIV RNA < 50 c/mL at W48, by ITT, LOCF ; non-inferiority if upper margin of a one-sided 95% CI for the difference = 10%, power 80%
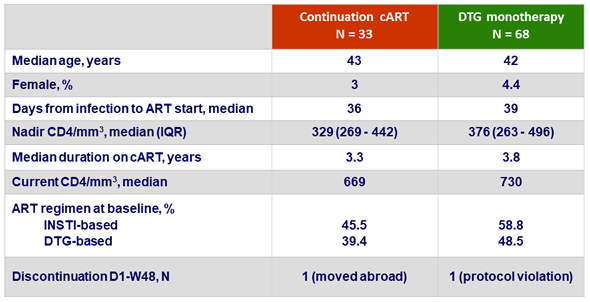
Baseline characteristics and patient disposition
HIV RNA < 50 c/mL at W48
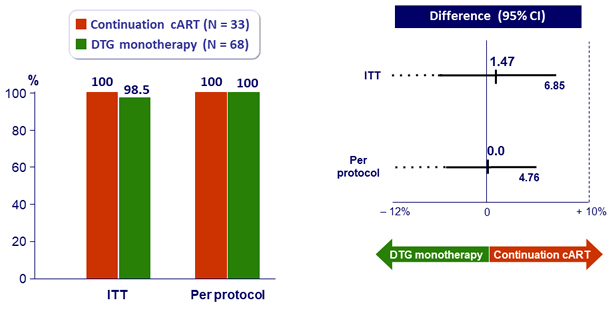
- Non inferiority achieved at W48
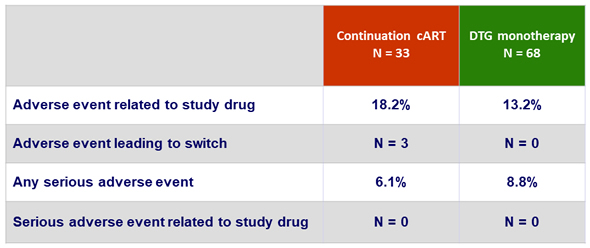
Adverse events