Hocqueloux L, Clin Infect Dis 2019 ; Janv 2 , Epub ahead of print
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG monotherapy
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG monotherapy
- Although DTG monotherapy was virologically non inferior at W24 to DTG/ABC/3TC as a switch strategy in virologically suppressed HIV-1 infected patients,
- the risk of virologic failure was significantly higher in DTG monotherapy vs DTG/ABC/3TC (n = 7 vs 0), with emergence of INSTI resistance,
- leading to recommendation against use of DTG monotherapy as a maintenance HIV strategy
Design
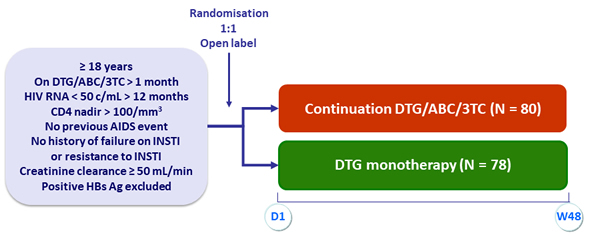
Objective
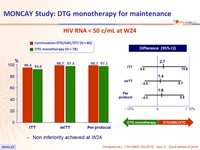
- Primary: % HIV RNA < 50 c/mL at W24, by ITT, missing or switch equals failure ; non-inferiority if upper margin of a two-sided 95% CI for the difference = 12%, power 90%
- sensitivity analyses: mITT, per-protocol
- Secondary: safety, virologic failure at W48
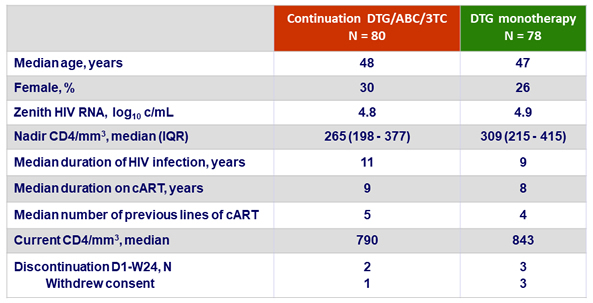
Baseline characteristics and patient disposition
HIV RNA < 50 c/mL at W24
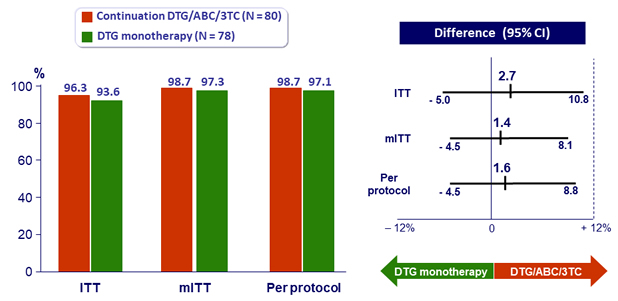
- Non inferiority achieved at W24
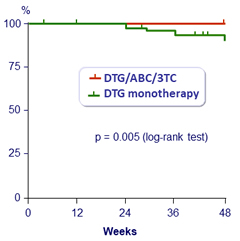
Virologic failures (2 consecutive HIV RNA > 50 c/mL) at W48 (Kaplan-Meier)
Virologic failures in the DTG monotherapy arm (N = 7) *
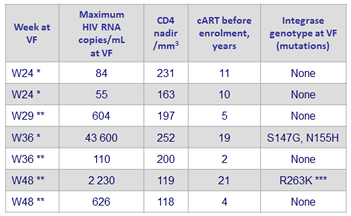
* Self-reported adherence at W4: 100%
** Self-reported adherence at W4: 95%
*** Presence also of a mutation in the 3’PPT region
- By W48: 7 virologic failures in DTG monotherapy arm vs 0 in DTG/ABC/3TC (trial was discontinued)
Adverse events
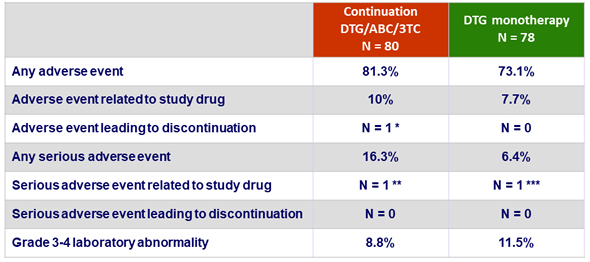
* Mood disturbance
** Grade 4 CK elevation
*** Spontaneous abortion