Gatell JM. AIDS 2017;31:2503-14 ; Gatell JM. Clin Infect Dis 2019 ; 68 :597-606
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG + FTC/TDF or ABC/3TC vs IP/r + FTC/FDT vs ABC/3TC
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG + FTC/TDF or ABC/3TC vs IP/r + FTC/FDT vs ABC/3TC
Drugs
DTG, DRV/r, LPV/r, 2 NRTI, FTC/TDF, ABC/3TC, TDF, ABC, FTC, 3TC
DTG, DRV/r, LPV/r, 2 NRTI, FTC/TDF, ABC/3TC, TDF, ABC, FTC, 3TC
- Over 48 weeks, in virologically suppressed patients with high cardiovascular risk (older than 50 years and/or with a Framingham score > 10%) and receiving triple therapy with PI/r + 2 NRTIs
- Switching to a DTG regimen was non-inferior. Sensitivity and subgroup analysis support this conclusion
- Improved total cholesterol and other lipid fractions in the overall population and in several subgroups
- Very few episodes of confirmed virological failure and no resistance mutations selected
- Overall tolerance was good and similar in both arms
Design
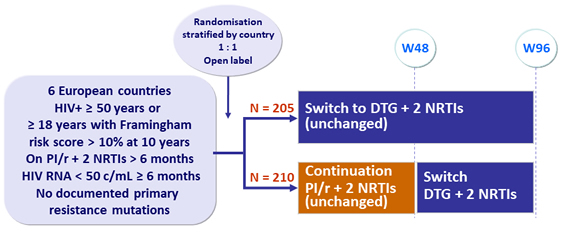
Primary endpoints
- Proportion of patients with virological success at W48 (no consecutive HIV-1 RNA > 50 c/mL and no treatment discontinuation): non-inferiority of DTG, by ITT, Kaplan-Meier analysis; lower limit of the 95% CI for the difference = - 10%, power 90%
- Mean percentage changes in fasting lipid total cholesterol at W48 (between treatment difference of 12%, 99% power)
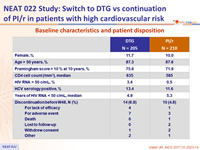
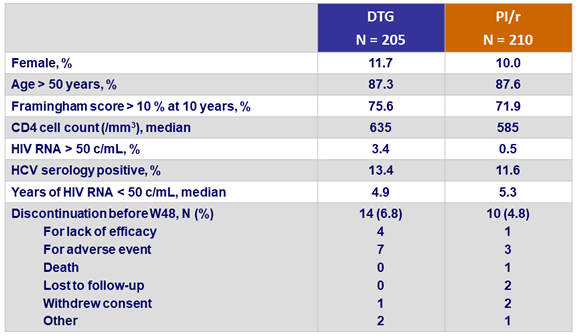
Baseline characteristics and patient disposition
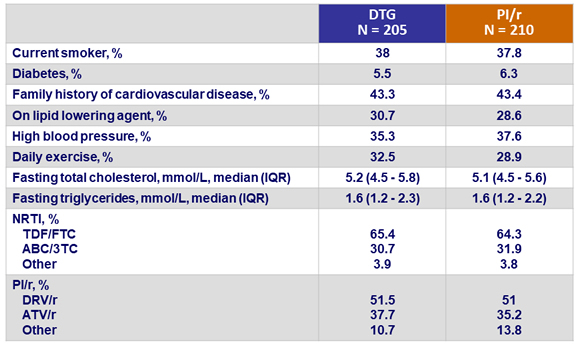
CV risk factors and ARV therapy at screening
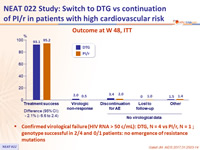
Outcome at W 48, ITT
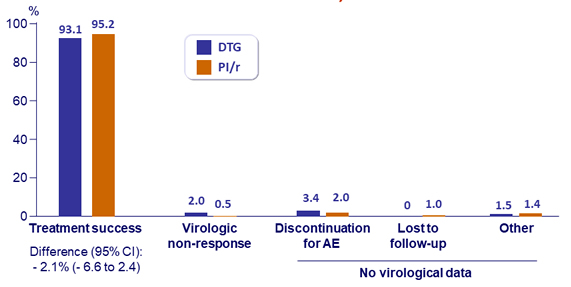
- Confirmed virological failure (HIV RNA > 50 c/mL): DTG, N = 4 vs PI/r, N = 1 ; genotype successful in 2/4 and 0/1 patients: no emergence of resistance mutations
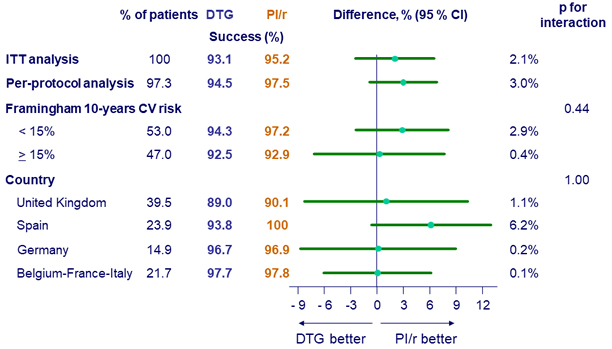
Treatment success at W48 (ITT, per-protocol and sub-groups)
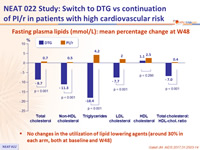
Fasting plasma lipids (mmol/L): mean percentage change at W48
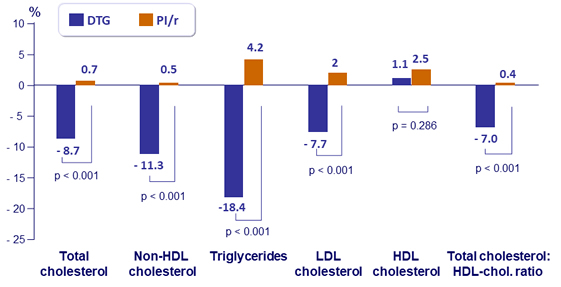
- No changes in the utilization of lipid lowering agents (around 30% in each arm, both at baseline and W48)
Adverse events, %
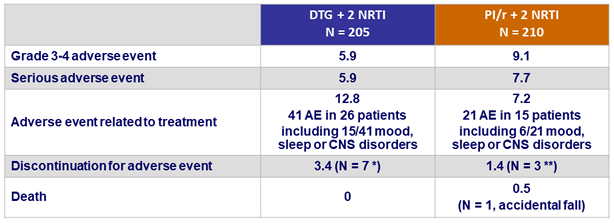
* Acute HCV infection (N = 1), mood and/or sleep disorders (N = 6)
** Acute HCV infection (N = 1), dyspepsia (N = 1), worsening of renal function (N = 1)
Median change in eGFR (CKD-EPI) at W48 (p < 0.001)
- DTG: ~ -8 ml/min
- PI/r: 0 ml/min
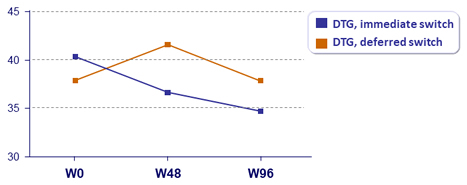
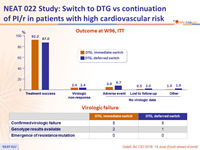
Outcome at W96, ITT
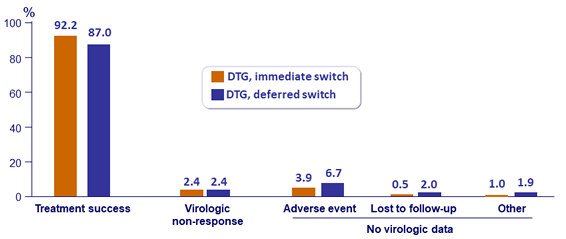
Virologic failure
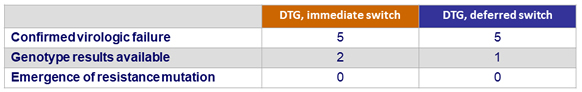
- Discontinuation for adverse event W48-W96
- Deferred switch, N = 9 (mood and/or sleep disorders, N = 7)
- Immediate switch: none
- Change in fasting lipids
- Deferred switch: improvement of lipid parameters at W96 (total cholesterol, non-HDL cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides), all statistically significant and of same level as at W48 in immediate switch group
- Immediate switch: stabilisation of improvement between W48 and W96
Change in % of patients receiving or requiring lipid lowering agents according to NCEP ATP-III guidelines