De Jesus E. Lancet HIV 2017; 4:e205-13
Switch studies in virologically suppressed patients
» Switch TDF to TAF
» EFV/FTC/TDF vs RPV/FTC/TAF
RPV, EFV, FTC/TAF, FTC/TDF, TAF, TDF, FTC
- Overall, virally suppressed, HIV-infected individuals who switched to rilpivirine, emtricitabine, and tenofovir alafenamide maintained viral suppression at 48 weeks similarly to those who remained on efavirenz, emtricitabine, and tenofovir disoproxil fumarate
- The rilpivirine, emtricitabine, and tenofovir alafenamide single-tablet regimen was well tolerated and associated with significant improvements in measures of bone and renal safety
Design
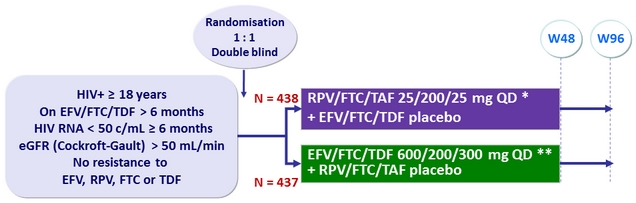
* With food, AM
** Without food, PM
Endpoints
- Primary: proportion of patients maintaining HIV RNA < 50 c/mL at W48 (ITT, snapshot) ; non-inferiority if lower margin of a two-sided 95.001% CI for the difference = - 8%, 95% power
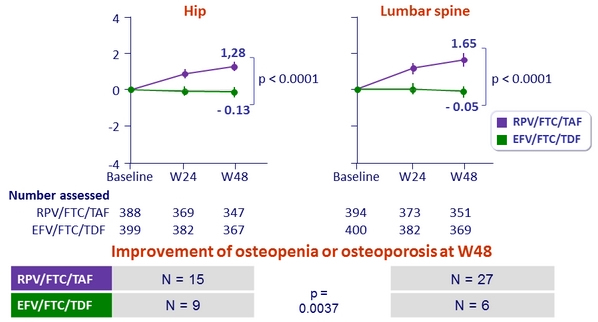
- Secondary: percentage change for hip and spine bone mineral density between treatment groups ; 95% power to detect a 1.38% difference
(non-inferiority margin) ; multiple adjustments to test for superiority
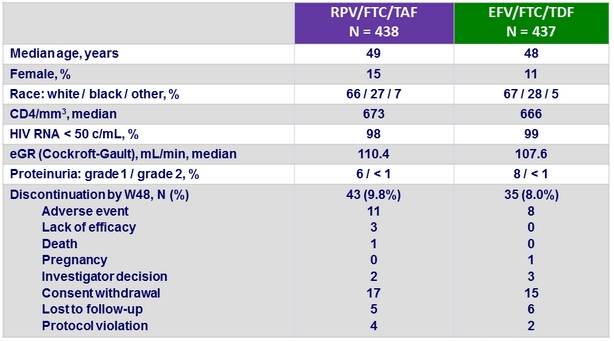
Baseline characteristics and patient disposition
Virologic outcome at W48 (ITT, snapshot)
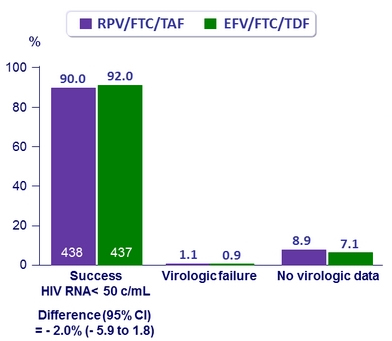
Other efficacy results at W48
- Per protocol analysis
(HIV RNA < 50 c/mL)
- 99.1% RPV/FTC/TAF
- 99.3% EFV/FTC/TDF
- Virologic success was similar between treatment groups for the subgroups of age, sex, race, and geographic region
- Virologic success lower in RPV/FTC/TAF group if adherence ≥ 95%: 91%
vs 95% for EFV/FTC/TDF - Mean changes in CD4/mm3
- + 23 RPV/FTC/TAF
- + 12 EFV/FTC/TDF
Resistance analysis
- Genotype and Phenotype testing if confirmed HIV RNA ≥ 50 c/mL and confirmatory sample ≥ 400 c/mL, or HIV RNA ≥ 400 c/mL at W48 or at the last visit on study drug
- 6 patients in the RPV/FTC/TAF group: no emergent resistance mutations ;
- 4 were re-suppressed without changing therapy
- 2 patients in the EFV/FTC/TDF group: emergence of resistance to FTC (M184V) and RPV (V106I/L + Y188L)
- Historical genotypes : resistance mutations to study drug
in 3 participants
- 2 patients in the RPV/FTC/TAF group (K103N ; E138A)
- 1 patient in the EFV/FTC/TDF group (K103N)
- All 3 discontinued at W36 or W48 with HIV RNA < 50 c/mL
Adverse events, %
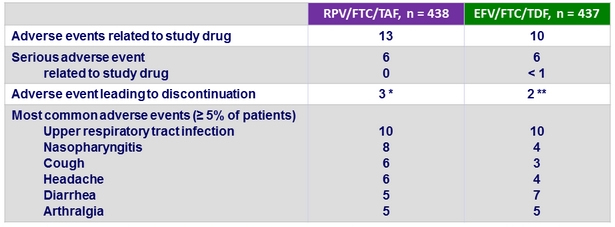
* Anaemia (N = 1), diarrhea (N = 1), vomiting (N = 1), constipation (N = 1), fatigue (N = 2), ulcer haemorrhage (N = 1), localised infection (N = 1), multiple fractures (N = 1), road traffic accident (N = 1), dysgeusia (N = 1), headache
(N = 1), somnolence (N = 1), anxiety (N = 1), cough (N = 1), decreased GFR (N = 1), generalised pruritus (N = 1)
** Atrial fibrillation (N = 1), diarrhea (N = 1), vomiting (N = 1), abdominal distension (N = 1), abdominal pain (N = 1), constipation (N = 1), dysphagia (N = 1), gastro-oesophageal reflux (N = 1), nausea (N = 1), hypersensitivity (N = 1), sinusitis (N = 1), arthralgia (N = 1), confusional state (N = 1), insomnia (N = 1), asthma (N = 1), rash (N = 1)
- 1 patient died from methamphetamine and cocaine overdose in the RPV/FTC/TAF group
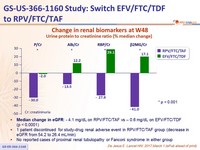
Change in renal biomarkers at W48
Urine protein to creatinine ratio (% median change)
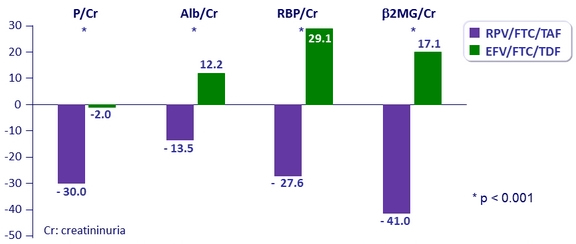
- Median change in eGFR : - 4.1 mg/ dL on RPV/FTC/TAF vs – 0.6 mg/ dL on EFV/FTC/TDF (p < 0.0001)
- 1 patient discontinued for study-drug renal adverse event in RPV/FTC/TAF group (decrease in eGFR from 54.2 to 26.4 mL/min)
- No reported cases of proximal renal tubulopathy or Fanconi syndrome in either group
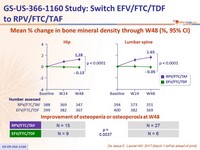
Mean % change in bone mineral density through W48 (%, 95% CI)
Fasting lipids changes at W48
- Decreases in total cholesterol, direct LDL, HDL and triglycerides in the RPV/FTC/TAF group
- Stable in the EFV/FTC/TDF group
- Change in total cholesterol:HDL-cholesterol ratio was similar in both groups
- Introduction of lipid-lowering agent between baseline and W48: 4% in both groups