Eron JJ. Lancet. 2010 Jan 30;375(9712):396-407
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to RAL-containing regimen
» RAL vs LPV/r
Switch studies in virologically suppressed patients
» Switch to RAL-containing regimen
» RAL vs LPV/r
Drugs
RAL, LPV/r
RAL, LPV/r
- In patients with virologic suppression on a LPV/r-containing regimen, switching from LPV/r to RAL was associated, at W24, with:
- Greater reductions in lipid concentrations than was continuation of LPV/r
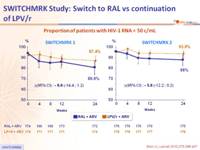
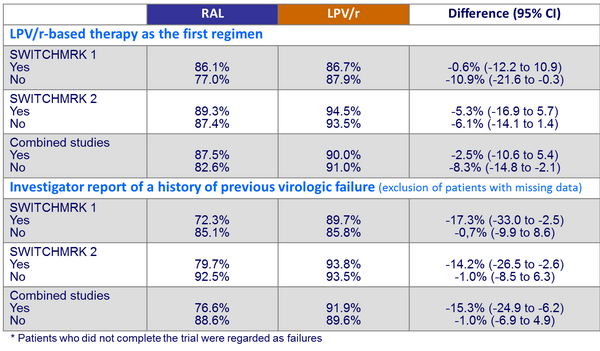
- Lower rate of HIV suppression, especially in patients who had a history of virologic failure before entry. Results did not establish non inferiority of RAL to LPV/r
- In the post-hoc analysis, patients without previous virologic failure had similar viral suppression rates in both treatment groups (switch to RAL or continuation of LPV/r)
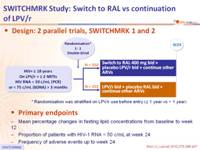
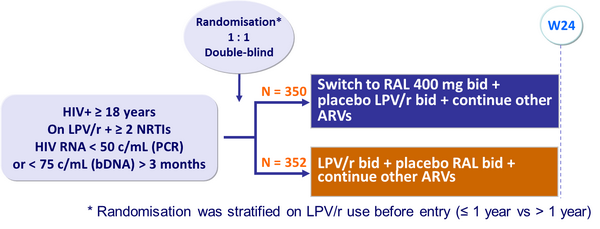
Design: 2 parallel trials, SWITCHMRK 1 and 2 :
Endpoints :
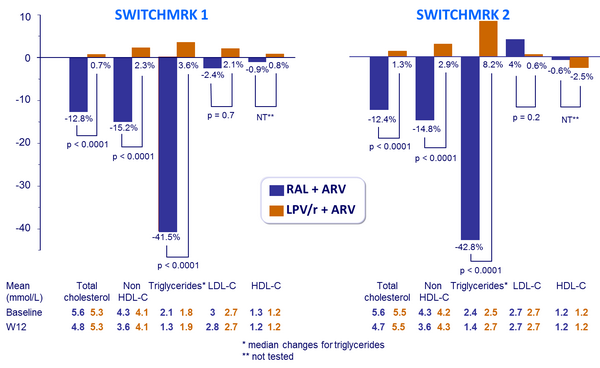
- Mean percentage changes in fasting lipid concentrations from baseline to week 12
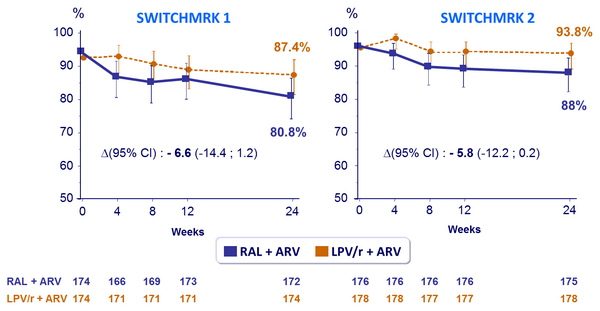
- Proportion of patients with HIV-1 RNA < 50 c/mL at week 24
- Frequency of adverse events up to week 24
Objectives :
- Lipids: 99% power to detect a between-treatment difference of 11%, 53% and 13% in the mean percentage change from baseline in total cholesterol, triglycerides, and non-HDL cholesterol, respectively, and 71% power to detect a between-treatment difference of 4% in the mean percentage change from baseline in LDL cholesterol
- Viral load: non inferiority of RAL vs LPV/r: % HIV-1 RNA < 50 c/mL at week 24 (lower limit of the 95% CI for the difference = - 12%, 90% power)
- Adverse events: for adverse events occurring in 20% of patients, each study had 80% power to declare with 95% confidence that the true difference between treatment groups was 12% or lower
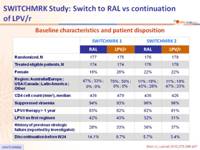
Design: 2 parallel trials, SWITCHMRK 1 and 2 :
Mean* % changes in fasting lipid concentrations from baseline to W12 :
Proportion of patients with HIV-1 RNA < 50 c/mL :
Proportion of patients with HIV-1 RNA < 50 c/mL at W24* :
Grade 3 or 4 laboratory abnormalities :
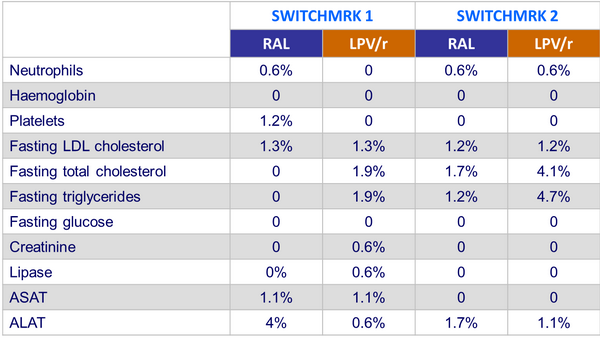
Safety, resistance data :
- Similar frequency of clinical and laboratory events in both groups
- No serious drug-related adverse event
- Diarrhoea of moderate to severe intensity: 3% in LPV/r group vs 0% in RAL group
- Discontinuation because of adverse events: 4 in LPV/r group vs 6 in RAL group
- 49 patients had confirmed virologic failure:
- 32 in the RAL group: for 27 (84%), LPV/r was not their first ARV regimen and 18 (67%) of these patients had a history of virologic failure on previous regimens
- 17 in the LPV/r group: for 8 (47%), LPV/r was not their first ARV regimen and 4 (50%) of these patients had a history of virologic failure on previous regimens
- Raltegravir-associated resistance mutations were found at failure in 8/11 assessable patients