Switch to BIC/FTC/TAF
GS-US-380-1844 Study : Switch to BIC/FTC/TAF
Original article : Molina JM. CROI 2018, Abs. 22
Last update :
11/05/2018
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- Switching to BIC/FTC/TAF was non-inferior to remaining on DTG/ABC/3TC, at W48
- No treatment emergent resistance
- Discontinuation for adverse event was rare: 2% on BIC/FTC/TAF and 1% on DTG/ABC/3TC at W48
- Study drug-related adverse events occurred with significantly higher frequency in DTG/ABC/3TC arm
- Increased grade 3-4 amylase was seen in 2% of BIC/FTC/TAF and no patient on DTG/ABC/3TC
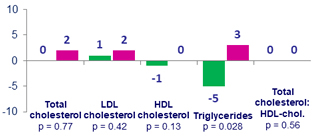
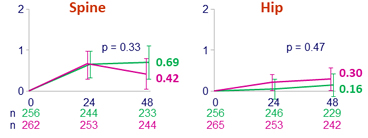
- The lipid, bone and renal parameters of switching to BIC/FTC/TAF were comparable to remaining on DTG/ABC/3TC through 48 weeks
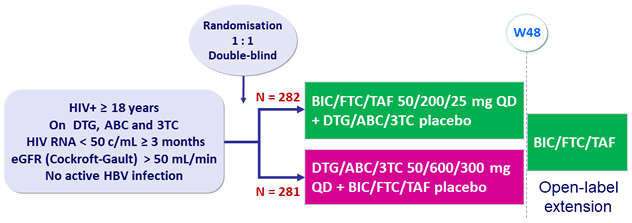
Design
Endpoints
- Primary: proportion of patients with HIV RNA ≥ 50 c/mL at W48
(ITT, snapshot) ; non-inferiority if upper margin of a two-sided 95.002% CI
for the difference = 4%
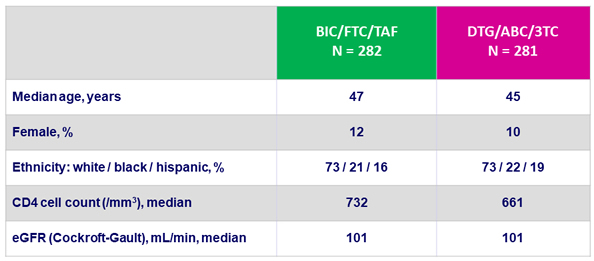
Baseline characteristics
Virologic outcome at W48
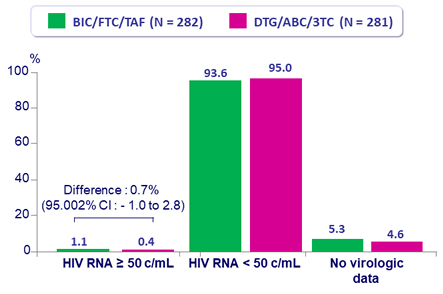
- No emergence of resistance in either group
Adverse events between D0 and W48, %
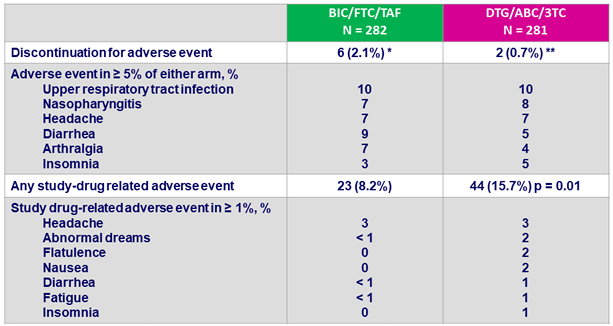
* Headache (N = 2), vomiting (N = 1), cerebrovascular accident (N = 1), abnormal dreams (N = 1),
suicidal ideation (N = 1) ; ** headache (N = 1), pruritus (N = 1)
Grade 3 or 4 laboratory abnormalities, N (%)
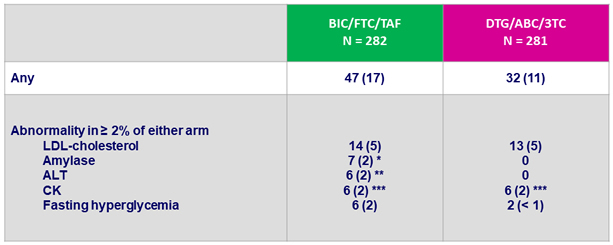
* All elevations transient and not associated with pancreatitis : lipase normal in 4/7
** Acute HCV infection (N = 3), acute HAV infection (N = 1), alcohol (N = 1), NASH (N = 1)
*** No case of rhabdomyolysis
Median percent change in quantitative proteinuria at W48
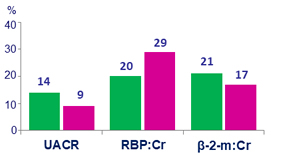
UACR: urine albumin:creatinine ratio
RBP: retinol-binding protein
β-2- m: beta-2 microglobulin
- Median change in eGFR CG at W48:
- 4.3 mL/min BIC/FTC/TAF vs
+ 0.2 mL/min DTG/ABC/3TC
(p < 0.001)
Median change in fasting lipids (mg/dL) at week 48
Mean % change in bone mineral density
 Back to Table of Contents
Back to Table of Contents



