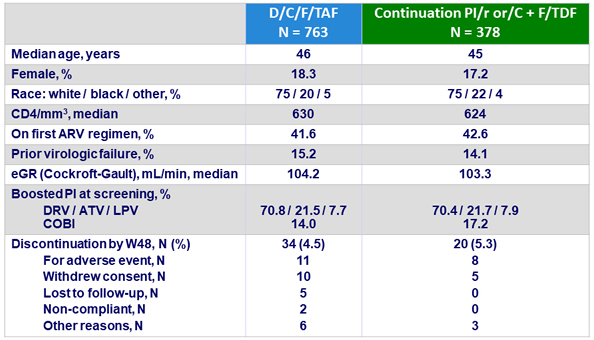
Baseline characteristics and patient disposition
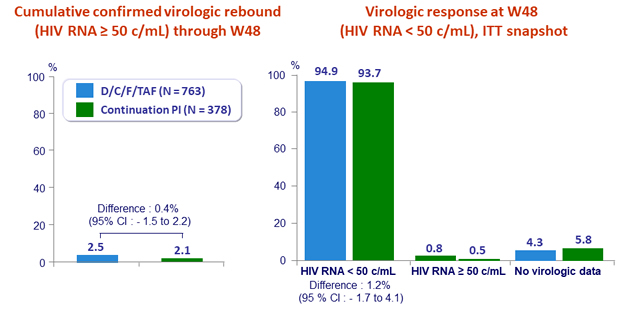
- 4 patients with virologic rebound genotyped: 1 in D/C/F/TAF (presence of D67D/N) and 3 in continuation group (E138E/G NNRTI mutation in 1)
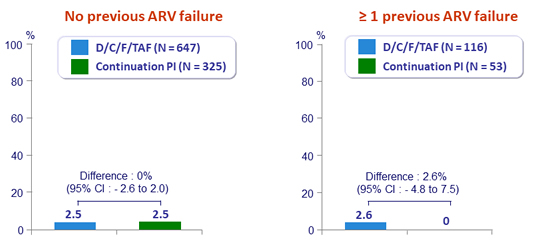
Virologic rebound rate through W48 according to previous antiretroviral failure
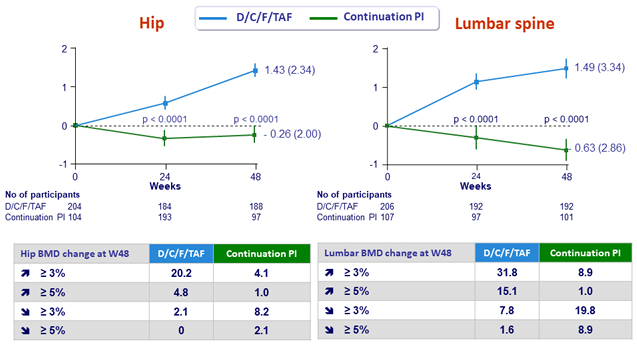
Mean (SE) % change from baseline in bone mineral density (g/cm²)
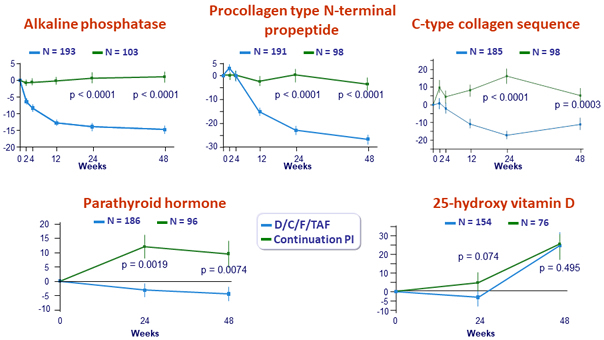
Mean (SE) % change from baseline in bone biomarkers
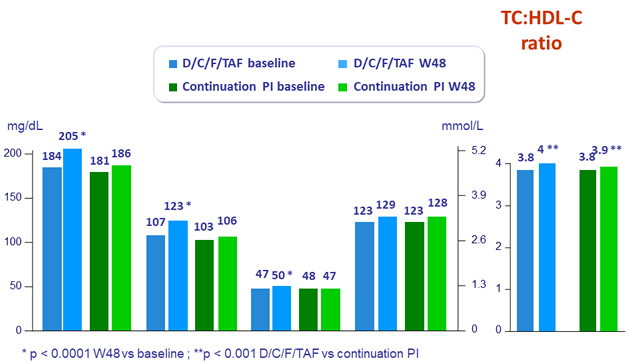
Median lipid values
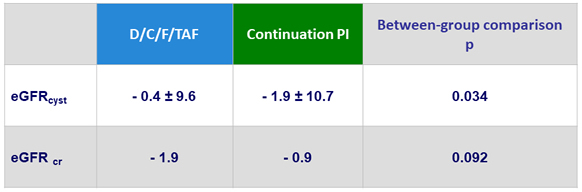
Mean Changes in eGFR (mL/min/1.73 m²) at W48
Adverse events between D0 and W48, %
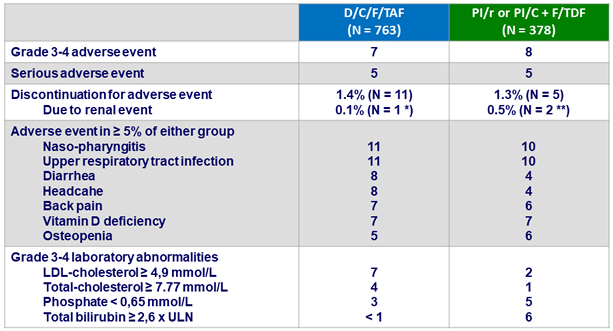
*
Worsening of pre-existing renal insufficiency ; ** Toxic nephropathy, N = 1 ; tubulopathy , N = 1



