NRTI-Sparing
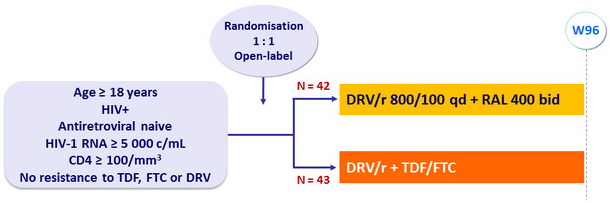
RADAR Study: DRV/r + RAL vs DRV/r + TDF/FTC
Original article : PLoS One. 2014 Aug 29;9(8):e106221 - RJ Bedimo
Last update :
18/07/2016
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- The NRTI-sparing regimen RAL+ DRV/r did not achieve similar week 48 virologic efficacy compared with TDF/FTC + DRV/r, but was better with regard to markers of bone health
- The two regimens achieved comparable immunologic response
- Patients in the TDF/FTC arm had smaller increases in total cholesterol
- Limitations
- Small sample size
- Unpowered to establish non-inferiority
- AEs self-reported, open-label unblinded design
- No site-specific BMD evaluation
Design
Objective
- Primary endpoint: time to loss of virologic response until W24 (ITT, TLOVR)
- Definition of failure: the earliest of any of the following events: death, permanent discontinuation of the study drug, loss to follow-up, or plasma
HIV-1 RNA concentrations > 48 copies/ml obtained at 2 consecutive visits or one value > 48 copies/ml followed by permanent discontinuation of the study drug or loss to follow-up
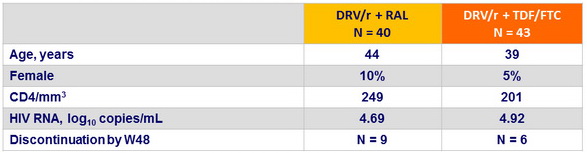
Baseline characteristics (mean), and disposition
Virologic efficacy
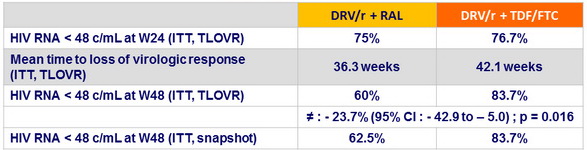
Resistance testing: no treatment-emergent resistance-associated mutations
Lipid parameters, renal function, body fat and bone mineral density
Mean changes from baseline (95% CI)
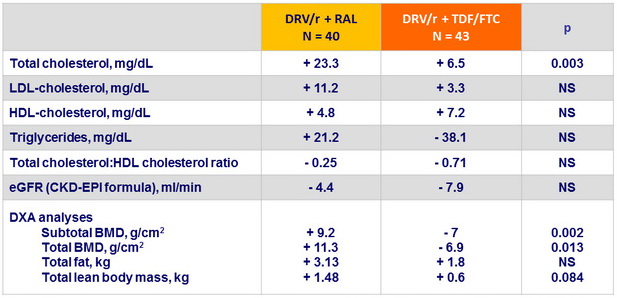
Grade 3 or higher clinical or laboratory adverse event: 5 in the RAL arm vs 2 in the TDF/FTC arm
 Back to Table of Contents
Back to Table of Contents



