NRTI-Sparing
SPARTAN Study: ATV + RAL BID vs ATV/r + TDF/FTC QD
Original article : HIV Clin Trials. 2012 May-Jun;13(3):119-30 - MJ Kozal
Last update :
09/10/2014
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- ATV + RAL BID achieved virologic response rate comparable to current standard of care for treatment-naïve subjects
- ATV + RAL was associated with
- Emergence of resistance to RAL in case of virologic failure
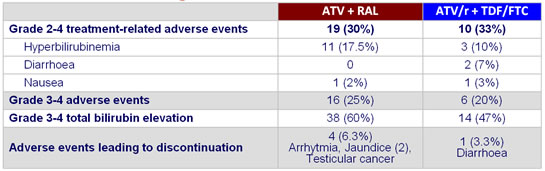
- Higher rates of severe hyperbilirubinemia compared with �ATV/r 300/100 mg QD
- Could be related to higher ATV exposure
- No new or unexpected safety signals
- Study was early terminated and regimen of ATV 300 mg BID �+ RAL 400 mg BID considered not to be optimal for further clinical development
Design :
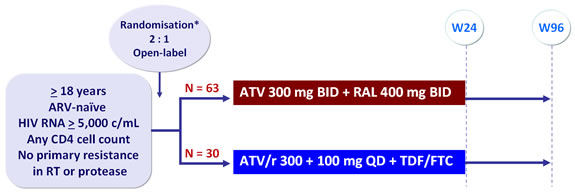
*Randomisation was stratified by HIV RNA (< or ≥ 100,000 c/mL)
Efficacy endpoint :
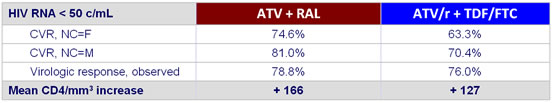
- Primary : HIV RNA < 50 copies/mL at week 24 by mITT �(confirmed virologic response (CVR) with non completers counted as failure)
- Other assessments : CVR with non completers counted as missing, �virologic response-observed
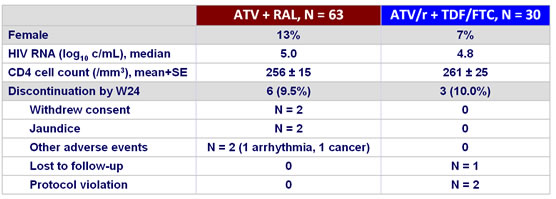
Baseline characteristics and patient disposition :
Response rate at week 24 :
Resistance data :
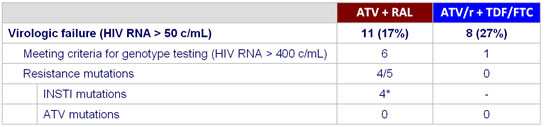
* N = 1 with Q148R, N = 1 with Q148Q/R + T97T/A, 2 with N155H
Adverse events through week 24 :
 Back to Table of Contents
Back to Table of Contents



