Comparison of INSTI vs EFV
SINGLE Study: DTG + ABC/3TC vs TDF/FTC/EFV QD
Original article : N Engl J Med. 2013 Nov 7;369(19):1807-18 - SL Walmsley, JAIDS 2015; 70:515-9 - SL Walmsley, ICAAC 2012. Abs.H556b - SL Walmsley, ICAAC 2014, Abs. H-647a - Pappa K & AIDS 2015; 29:2459-64 - Tebas P
Last update :
01/12/2015
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- DTG + ABC/3TC QD had a better safety profile and was superior through 48 weeks to TDF/FTC/EFV for first-line antiretroviral therapy
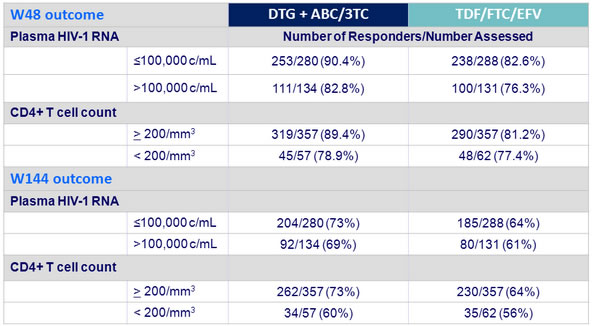
- Superior virologic response with DTG + ABC/3TC also seen in key demographic subgroups and in patients with low or high baseline viral load
- Statistical superiority in CD4 response for DTG + ABC/3TC
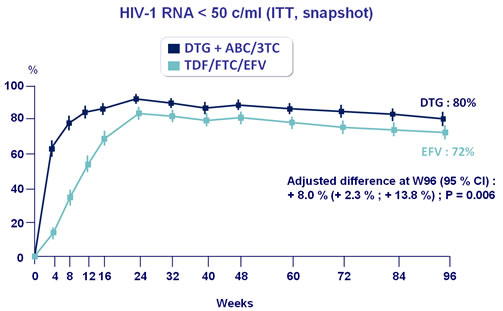
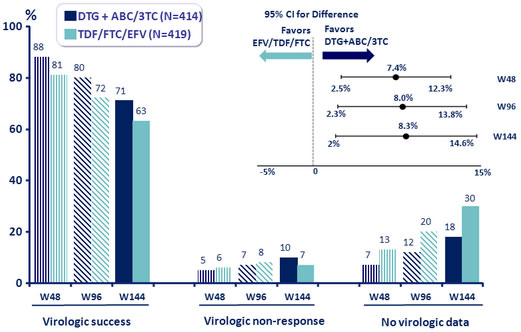
- Virologic superiority of DTG + ABC/3TC confirmed at W96 and W144
- No INSTI major mutations were detected through 144 weeks with DTG
- Lower occurrence of adverse events leading to discontinuation with DTG : 2% vs 10% at W48 ; 4% vs 14% at W144
- Significant lower reported rates of neuro-psychiatric and rash events with DTG + ABC/3TC
- Except for insomnia (15% vs 10% at W48)
-
Design :
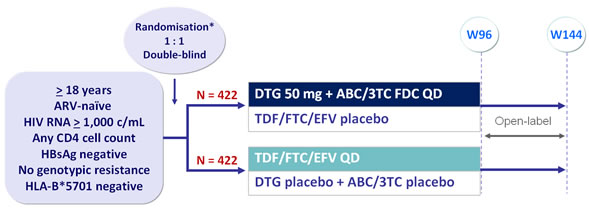
*Randomisation was stratified by HIV RNA (< or > 100,000 c/mL) and CD4/mm3 (< or > 200) at screening
Objective :
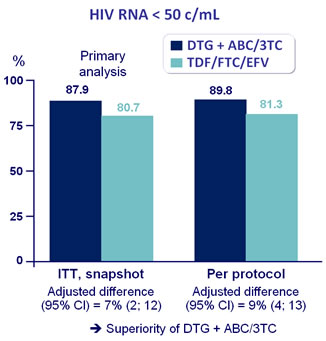
- Non inferiority of DTG at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (1-sided significance level of 2.5%, lower margin of �the 95% CI for the difference = -10%, 90% power)
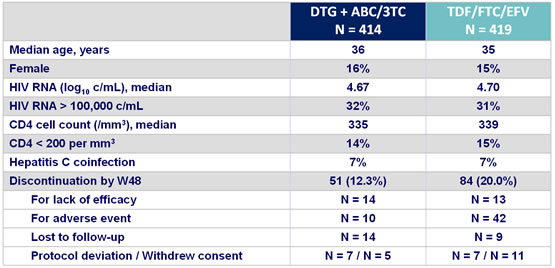
Baseline characteristics and patient disposition :
Response to treatment at week 48 :
Differences in viral suppression were also seen in key demographic subgroups including race, sex, �age and patients with �HIV RNA > 100,000 c/mL at baseline
Adjusted mean CD4/mm3 increase at W48 :
+ 267 for DTG + ABC/3TC
+ 208 for TDF/FTC/EFV (p<0.001)
Response to treatment at week 96 :
HIV-1 RNA < 50 c/mL at W144, ITT snapshot
HIV-1 RNA < 50 c/mL by baseline stratification factors
Virologic failure definition
- 2 consecutive plasma HIV-1 RNA > 50 c/mL, on or after W24
Criteria for resistance testing
- All patients with protocol defined virologic failure (PDVF)
Genotype of RT and integrase on baseline and suspected virologic failure samples
Resistance data at PDVF
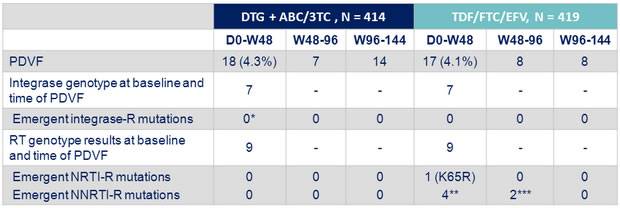
* E157Q/P polymorphism in 1 patient with no change in phenotype
** N = 1 with K101E, N = 1 with K103N, N = 1 with G190A, N = 1 with K103N + G190A ; **** N = 2 with K103K/N
Adverse events and laboratory abnormalities at week 48 :
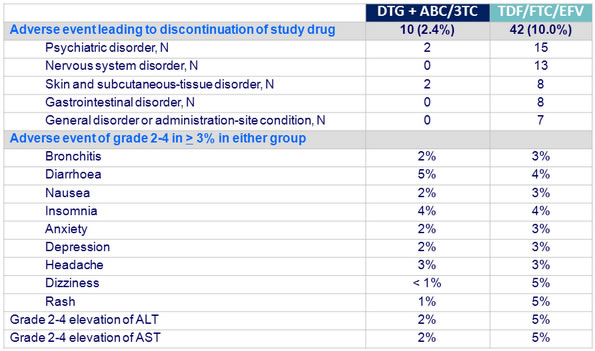
Insomnia was more frequent in DTG group at W48 (15% vs 10%)
Mean change in creatinine at W48 on DTG: + 0.12 to 0.15 mg/dL �(10.56 to 13.2 mmol/L), peak at W2, then stable
Serious adverse events related to study drug at week 48
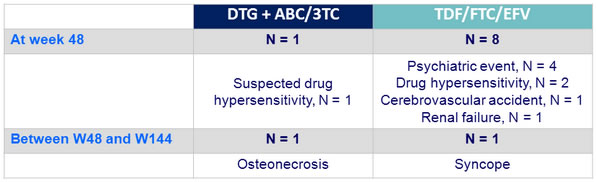
« ABC HSR » was reported between W0 and W96 in 2 patients in the DTG + ABC/3TC arm vs 5 in the TDF/FTC/EFV arm
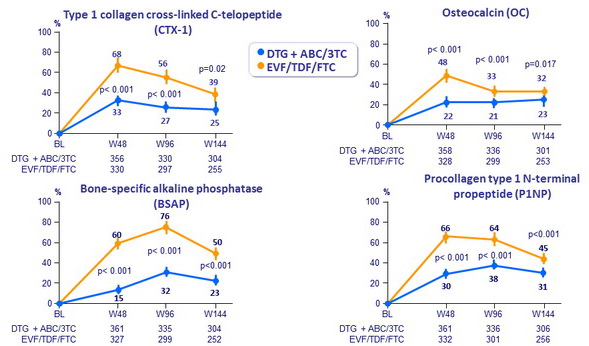
Mean change from baseline in bone turnover markers (%)
 Back to Table of Contents
Back to Table of Contents



