Comparison of INSTI vs INSTI
Study GS-US-380-1489: BIC/F/TAF QD vs DTG/ABC/3TC QD
Original article : Gallant J. Lancet. 2017 Nov 4;390(10107):2063-2072.
Last update :
31/01/2018
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- Initial HIV-1 therapy with BIC/F/TAF was non inferior to DTG/ABC/3TC �at W48 by snapshot algorithm
- 92.4% of patients on BIC/F/TAF and 93.0% of patients on DTG/ABC/3TC had HIV-1 RNA < 50 copies/mL
- Sensitivity analyses confirmed non inferiority
- No treatment emergent resistance
- BIC/F/TAF was well tolerated, with no adverse events leading to discontinuation
- Nausea was reported significantly more frequently in patients treated with DTG/ABC/3TC (p < 0.001)
- Gastrointestinal, neuropsychiatric, and sleep-related symptoms were reported more frequently in patients treated with DTG/ABC/3TC
- Changes from baseline in bone mineral density, lipid parameters and renal markers were comparable between treatment arms
Design
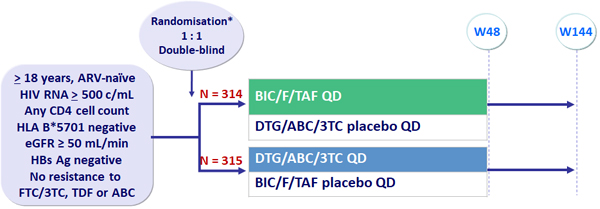
* Randomisation was stratified by HIV RNA (< 100 000 c/mL, 100 000-4000 000 c/mL or > 100 000 c/mL), CD4 (< 50/mm3, 50-199/mm3 or ≥ 200/mm3) at screening and geographic region (USA vs non-USA)
BIC/F/TAF : 50/200/25 mg, as STR
Objective
- Non inferiority of BIC/F/TAF at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (lower margin of the 2-sided 95.002% CI for the difference= -12%, 95% power)
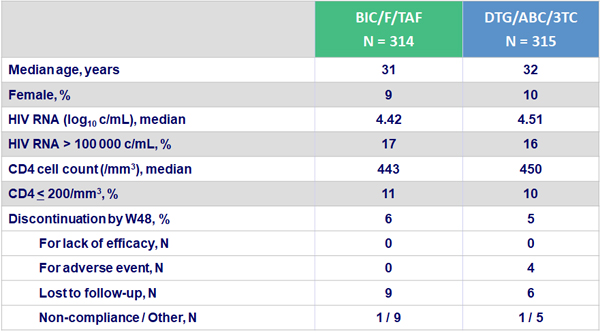
Baseline characteristics and patient disposition
Virologic outcome at week 48
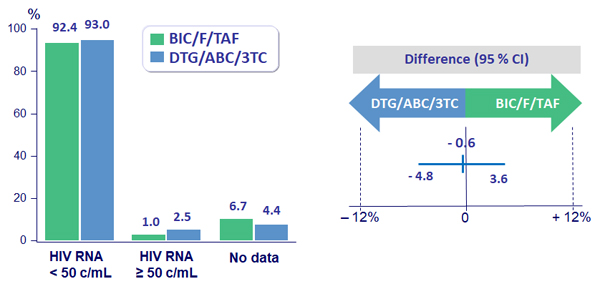
- HIV RNA < 50 c/mL (per-protocol)
- BIC/F/TAF: 99.3%
- DTG/ABC/3TC: 98.6%
- Met criteria for resistance testing (HIV RNA ≥ 200 c/mL)
- BIC/F/TAF: 1 vs DTG/ABC/3TC: 4
- No resistance emergence
- Mean CD4 increase at W48
- BIC/F/TAF: + 233/mm3
- DTG/ABC/3TC: + 229/mm3
Adverse events
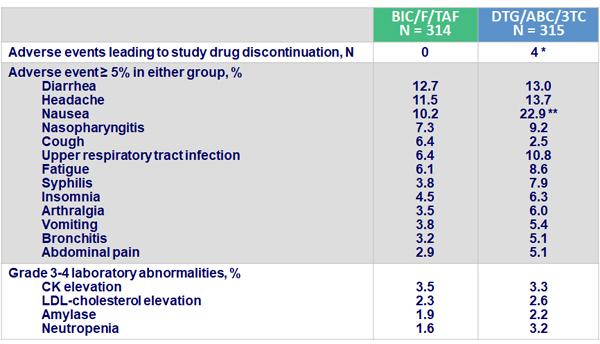
* Nausea, rash ; thrombocytopenia ; chronic pancreatitis, steatorrhea ; depression
** p < 0.001
Renal parameters, bone mineral density and lipid changes at W48
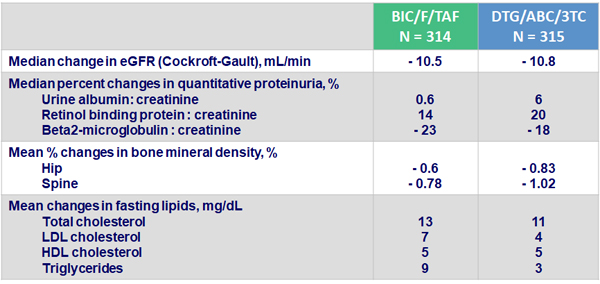
- None of the differences between groups were significant
- No discontinuations due to renal adverse events and no proximal tubulopathy in either arm
Steady-state pharmacokinetic parameters of BIC/F/TAF (N = 17)
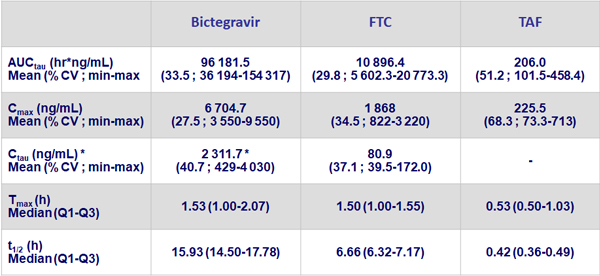
* BIC mean Ctau about 14 times higher than the protein adjusted effective concentration (162 ng/mL) against wild type HIV-1 virus.
 Back to Table of Contents
Back to Table of Contents