Comparison of NNRTI vs PI/r
DRIVE-FORWARD Study : DOR + 2 NRTI vs DRV/r
+ 2 NRTI
Original article :
Molina JM, Lancet HIV 2018, March 25 (Epub ahead of print)
Last update :
11/05/2018
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- DOR 100 mg QD, in combination with either TDF/FTC or ABC/3TC
- Achieved high virologic success at week 48
- And was non-inferior to DRV/r + 2 NRTI regardless of baseline HIV RNA
- Resistance mutations through 48 weeks
- None were detected in protocol-defined virologic failures
- Only 1/383 participants on DOR developed genotypic and phenotypic resistance to DOR + FTC/3TC
- Adverse events leading to discontinuation occurred with low frequency for both DOR and DRV/r
- Low rate of discontinuation due to rash or neuropsychiatric adverse events
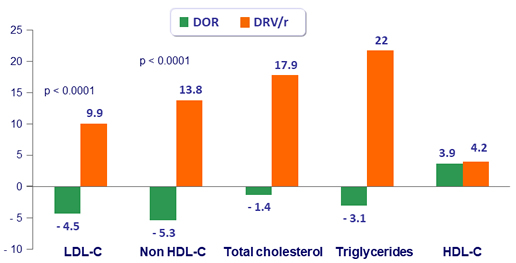
- Lipid changes were less pronounced for DOR than for DRV/r
- Once-daily DOR in combination with fixed-dose NRTIs represents an effective treatment option for HIV-1-infected, treatment-naive patients
Design
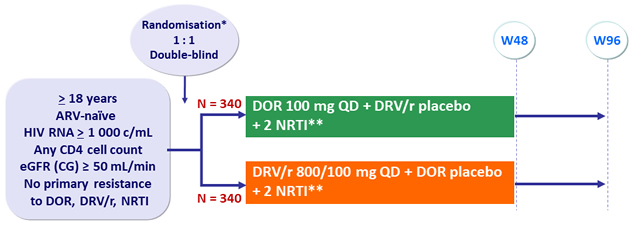
* Randomisation (DOR vs DRV/r) was stratified by HIV RNA (≤ or > 100 000 c/mL) at screening and NRTI backbone
** NRTI backbone (TDF/FTC or ABC/3TC if exclusion of the HLA-B*5701 allele) was selected by investigator
Objective
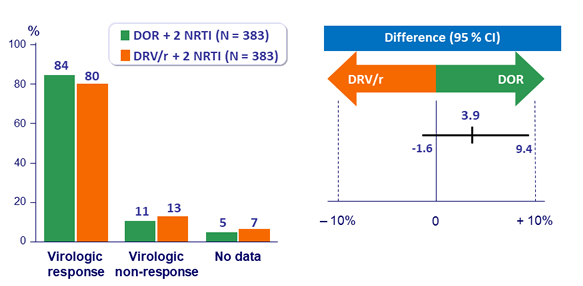
- Non inferiority of DOR at W48: % HIV RNA < 50 c/mL by intention to treat, �non completer = failure, snapshot analysis (lower margin of the 95% CI for the difference = - 10%, 90% power)
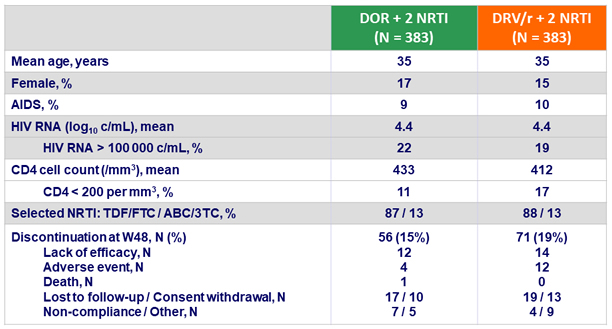
Baseline characteristics and patient disposition
Primary endpoint: HIV RNA < 50 c/mL at W48 (ITT, snapshot)
CD4 increase at W48 (ITT, NC = F)
- DOR: + 193/mm3
- DRV/r: + 186/mm3
HIV RNA < 50 c/mL, observed failure approach *
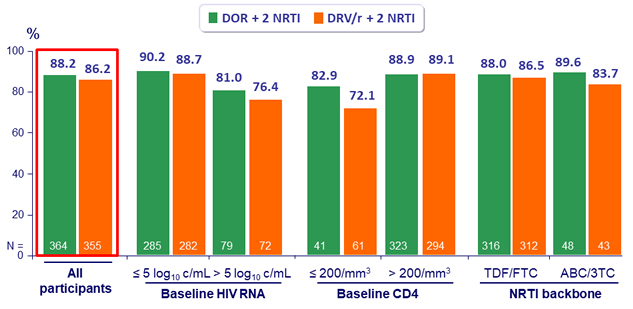
* Discontinuation due to lack of efficacy counted as failures, data missing for other reasons excluded
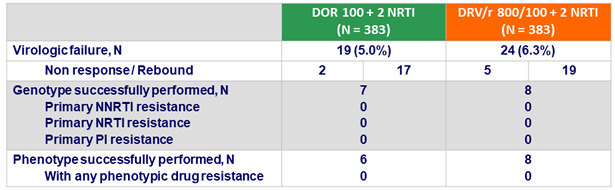
Protocol-defined virologic failures (PDVF)
Definition
- Non response: HIV RNA ≥ 200 c/mL at W24 or W36 or confirmed HIV RNA ≥ 50 c/mL at W48
- Rebound: confirmed HIV RNA ≥ 50 c/mL after obtaining HIV RNA < 50 c/mL
Resistance tests
- (genotype and phenotype) performed on confirmatory sample
if HIV RNA > 400 c/mL
Emergence of drug resistance in participants with discontinuations
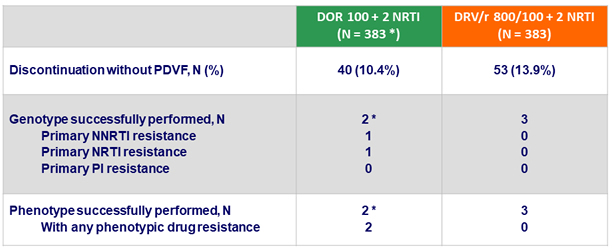
*
1 discontinuation for non-compliance at W24, with emergence of resistance to DOR (V106I + H221Y ; > 90 fold increased IC50) and FTC (M184V) ; 1 discontinuation for rash at W2, with increased DOR IC50 2.8 fold WT (resistance cutoff = 2.5 fold), but no genotypic resistance mutations
Adverse events (AE), %
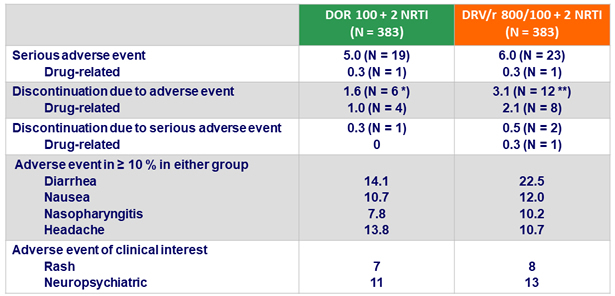
* Death = 1, rash = 2, nausea = 2, abdominal pain = 1, kidney injury = 1
** Abdominal pain = 2, diarrhea = 1, nausea = 1, flatulence = 1, hiatus hernia = 1, ALT and AST increase = 2, hepatitis B or C = 2, peripheral edema = 1, pyrexia = 1, rash = 1, tuberculosis = 2
Fasting lipids, changes from baseline at W48 (mg/ dL )
 Back to Table of Contents
Back to Table of Contents



