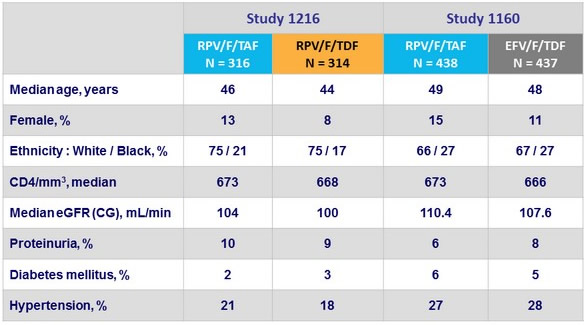
Baseline characteristics
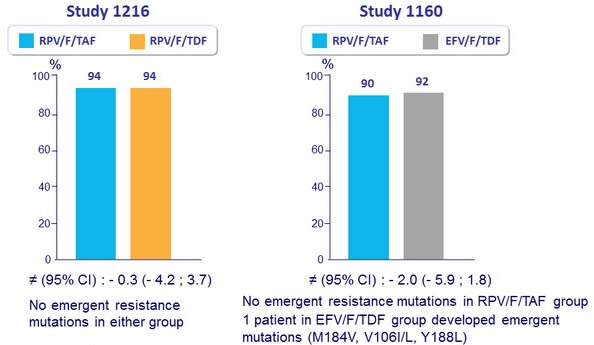
HIV-1 RNA < 50 c/mL at W48 (ITT, FDA snapshot)
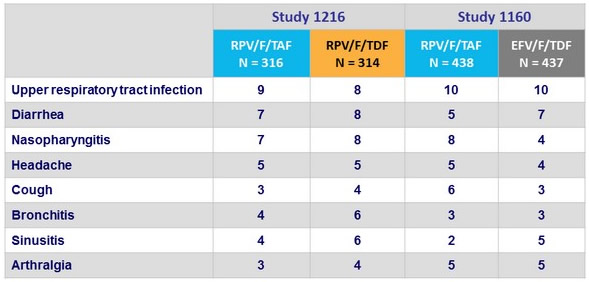
Adverse events, % (≥ 5% in either group)
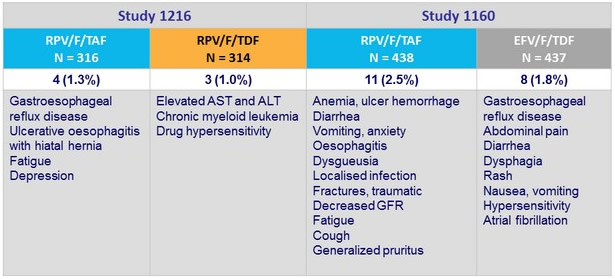
Adverse events leading to discontinuation, N (%)
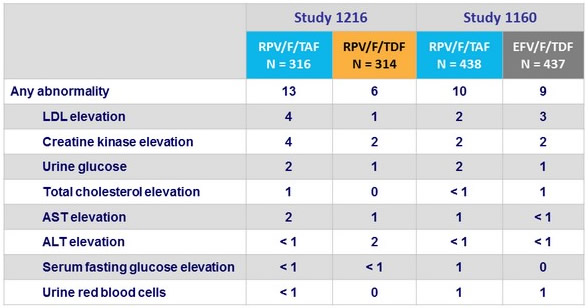
Grade 3-4 laboratory abnormalities, %
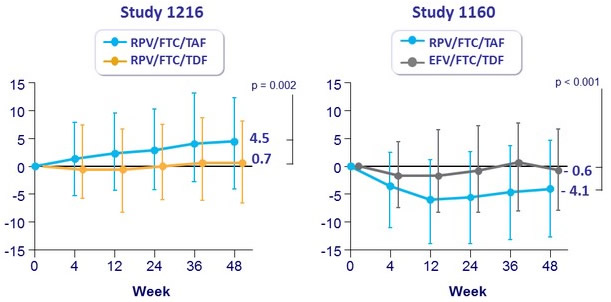
Median change in eGFR [ Cockroft-Gault ] (mL/min) at W48 (Q1-Q3)
Change in Proteinuria at W48 (median % change)
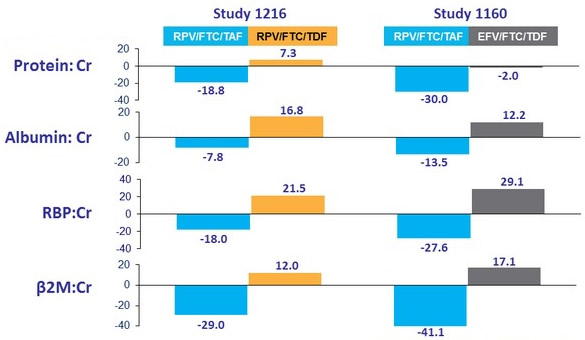
- All differences (RPV/F/TAF vs RPV/F/TDF or EFV/F/TDF) : p < 0.001 ; no reported cases of tubulopathy
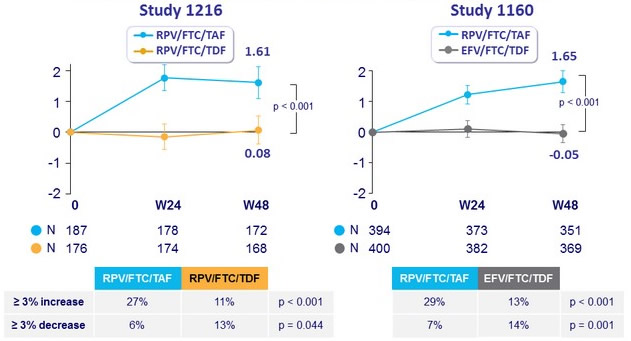
Median % change in Hip BMD through W48 (95% CI)
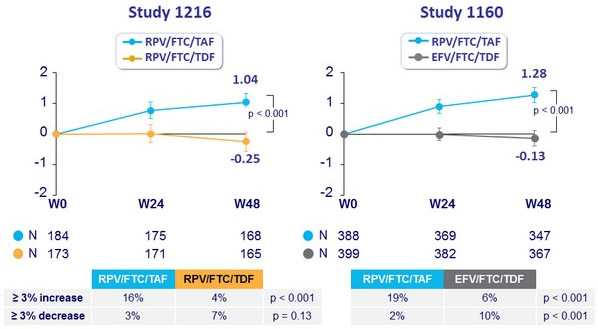
Median % change in Spine BMD through W48 (95% CI)
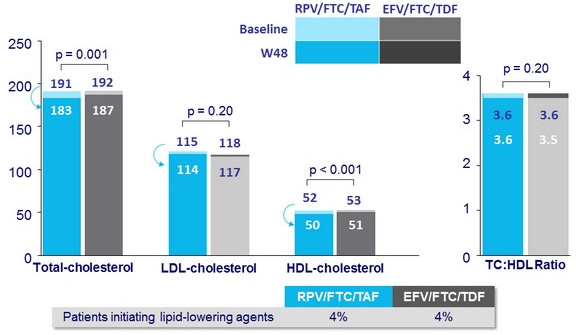
Median fasting lipids (mg/dL) at baseline and W48
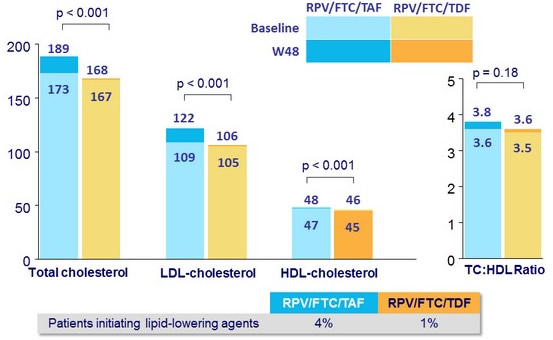
Median fasting lipids (mg/dL) at baseline and W48