Switch studies in virologically suppressed patients
Switch to ATV/r monotherapy
ACTG A5201
Original article : J Infect Dis. 2009 Mar 15;199(6):866-71 – TJ Wilkin
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- Limited pilot study, no control arm
Design :
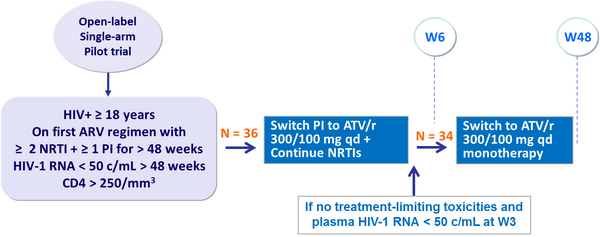
Primary endpoint :
- Time to virologic failure (2 consecutive HIV-1 RNA ≥ 200 c/mL)
- 85% power to detect a difference between W24 success rate of 75% with ATV/r alone vs a nominal rate of 90%
- Prior PI therapy
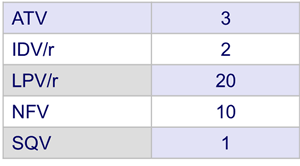
- Median CD4 cell count/mm3: at inclusion = 616 ; nadir = 253
- Kaplan-Meier estimate of the probability of virologic success�at W48 = 0.88 (lower limit of the 90% 1-sided CI: 0.81)
- In the 4 subjects with confirmed virologic failure there were no major PI-resistance mutations detected
- Grade 3 or 4 elevation of total biliribun: 17/34 subjects
 Back to Table of Contents Back to Table of Contents
|



