Switch studies in virologically suppressed patients
Switch to ATV/r monotherapy
OREY
Original article : Pulido F, EACS 2009;Abs. PS4/6
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- Pilot study of ATV/r monotherapy showing maintenance of virologic suppression in 79% of patients
Design :
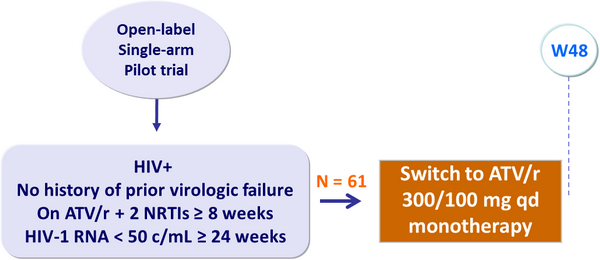
Primary endpoint :
- Proportion of patients with virologic rebound (HIV-1 RNA ≥ 400 c/mL)�or treatment discontinuation by W48
- Mean duration of prior ATV/r therapy: 84 weeks
- Use of PI prior to ATV/r: 59%
- Median CD4 cell count/mm3 at inclusion: 514
Outcome at Week 48
- Treatment failure, N = 13: 21% (95% CI: 11.9 -33.7%)
- Virologic rebound ≥ 400 c/mL, N = 7: 12%
- Virologic rebound ≥ 50 c/ml, N = 16: 27%
- Maintenance of HIV-1 RNA suppression < 50 c/mL: 67%
- Genotype at virologic rebound > 400 c/ml, N = 7 ; Major PI resistance in 1/7 + 1 post W48
- Grade 3 or 4 total bilirubin: 64%
- AE leading to discontinuation, N = 1
 Back to Table of Contents Back to Table of Contents
|



