Switch studies in virologically suppressed patients
Switch to DRV/r reduced dose
PROTEA Study: Switch PI or NNRTI to DRV/r QD monotherapy
Original article :
AIDS. 2015 Sep 10;29(14):1811-20 - A Antinori
Last update :
01/10/2015
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- In patients with virologic suppression on standard first-line triple therapy (2 NRTIs + 1 NNRTI or 1 PI), once-daily DRV/r monotherapy did not show non inferior HIV RNA suppression at week 48 compared with a standard therapy of 2 NRTIs + once-daily DRV/r
- A low nadir CD4 count (< 200/mm3) was highly predictive of treatment failure in the monotherapy arm.
- Two patients in the monotherapy arm with CD4 nadir < 200/mm3 developed viremia in both CSF and plasma, with one symptomatic case
- There was no difference in neurocognitive function or the risk of neuropsychiatric adverse events between DRV/r monotherapy and triple therapy
Design
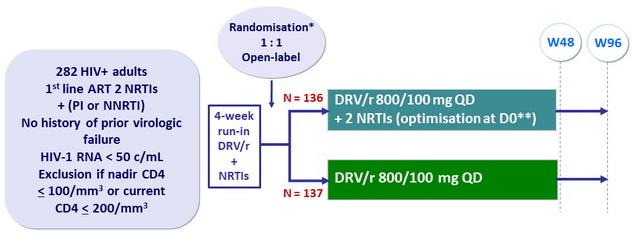
* Randomisation was stratified on HCV antibody status (+ or -)
** TDF, ABC or ZDV + 3TC or FTC
Objective
- Non inferiority in the proportion of patients with HIV-1 RNA < 50 c/ mL at W48 (ITT analysis, missing/discontinuation/switch= failure, snapshot algorithm ); lower limit of the 95% CI for the difference= - 12%, 80% power
- CNS substudy : CSF HIV RNA at baseline and W48
- Neurocognitive function using a series of neuropsychological tests
Baseline characteristics and patient disposition
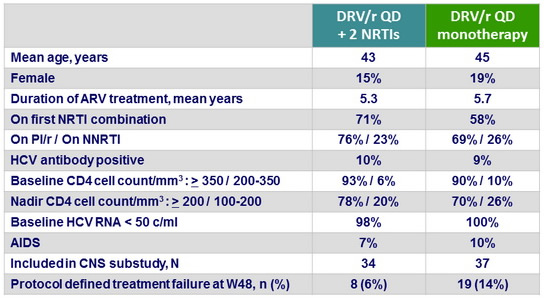
- At baseline, 8 patients had a nadir CD4 < 100/mm3 (5 in the monotherapy arm
and 3 in the triple therapy arm), and were excluded from the Per Protocol population
HIV RNA < 50 c/ mL at W48 (FDA snapshot analysis)
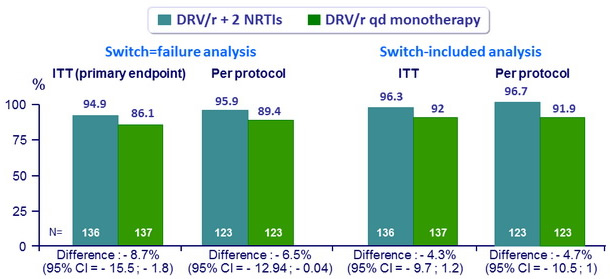
- DRV/r monotherapy is not non inferior to DRV/r + 2 NRTI
- Primary analysis adjusting for treatment group, HCV status, nadir CD4 and previous PI use : DRV/r QD mono non inferior to triple therapy (≠ - 5.8%, 95% CI: - 11.51 to - 0.14), but difference still inferior statistically
HIV RNA < 50 c/mL at W48 (FDA snapshot analysis) by baseline nadir CD4 cell count
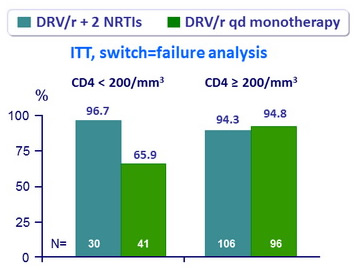
- Predictors of treatment failure (multiple regression model) :
- Low nadir CD4 (p = 0.005)
- Previous use of PI (p = 0.004)
- Genotype (3 patients with confirmed HIV RNA > 400 c/mL, 2 in monotherapy arm, 1 in triple therapy arm)
- No emergence of primary PI mutation
Safety
- Most common adverse events : infections or infestations (32%) and gastrointestinal (16%)
- Serious adverse events, N = 14 (5%): 9 in the monotherapy arm and 5 in the triple therapy arm. One unrelated death in the monotherapy arm
- Grade 2-4 adverse events considered treatment-related
- More common in the monotherapy (N = 12; 9%) than in the triple therapy arm (N = 2; 1%)
- In the monotherapy arm, these were mainly gastrointestinal events and rises in cholesterol after discontinuation of TDF
- Discontinuation of DRV for adverse event
- N = 5 (4%) in the monotherapy arm, N = 1 (1%) in the triple therapy arm
- Neurological adverse events, N = 27 (10%) :
- 13 in the monotherapy arm and 14 in the triple therapy arm
- Most common AE = headache (N = 14)
- 1 case of encephalomyelitis in the monotherapy arm :
- required hospitalization; HIV RNA detectable in plasma and CSF; re-suppressed and symptoms resolved after intensification with NRTIs including high-dose ZDV. NB : Nadir CD4 = 17/mm 3
Neurocognitive function and CNS substudy
- Improvement of all neurocognitive scores at W48 in both groups
(learning effect)
- No difference between arms in the global score (NPZ-5) over time
- W48 NPZ-5 score was very siginificantly associated to sex, race, and baseline NPZ-5 score (p < 0.0001). Alcohol consumption, smoking, history of cardiovascular events and age were also significantly associated. No effect of baseline HIV RNA, baseline CD4 count or nadir CD4.
- CNS substudy
- At baseline, HIV RNA < 50/ mL in the CSF in all patients
- At W48 : CSF HIV RNA < 50 c/ mL in all except 1 patient in the monotherapy arm : HIV RNA 654 c/mL , no symptoms, nadir CD4 : 166/mm3
- Mean CSF neopterin concentration ( nmol /L)
- Monotherapy : 4.8±2.1 at baseline vs 6.2±4.3 at W48
- Triple therapy: 4.8±1.3 at baseline vs 4.1±1.2 at W48
- Mean CSF albumin : normal range at W48 for both arms
 Back to Table of Contents Back to Table of Contents
|