Switch to PI/r monotherapy
MOBIDIP Study
Original article : Ciaffi L,
Lancet HIV 2017; 4:e384-92
Last update :
28/09/2017
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- After viral suppression with a second-line cART of PI/r plus 2 NRTIs, maintenance with PI/r plus 3TC is associated with
- A higher rate of success than PI/r monotherapy despite the presence of M184V mutation
- Significant more virological failures with PI/r (24.8% vs 3.0%)
- A similar CD4 response and adherence
- No differences in safety outcomes
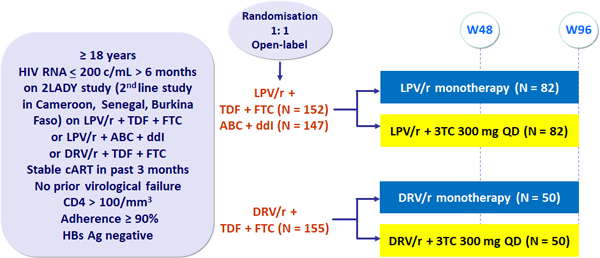
Design
Objective
- Primary endpoint: failure rate at W96 by ITT, defined as 1) a confirmed HIV �RNA > 500 c/mL, 2) reintroduction of the NRTI backbone or 3) interruption of the PI
- March 2016: Monotherapy arm discontinued following DSMB meeting
Baseline characteristics
and primary outcome at W48
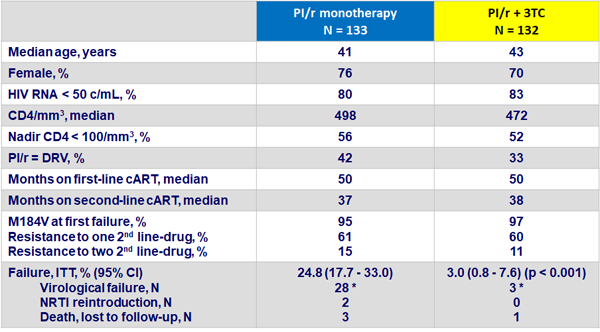
* All failures resuppressed to HIV RNA < 200 c/mL a median of 10 weeks after NRTI reintroduction
Other results
- Failure not associated with
- Adherence
- Nadir CD4 count
- PI
- CD4 gain similar in both groups at W48
- No differences in safety (PI/r monotherapy vs PI/r + 3TC)
- Severe adverse events = 11% (13% vs 10%)
- AIDS-defining events = 3% (5% vs 2%)
- No treatment interruptions for intolerance
- Laboratory parameters : no differences
- Changes in eGFR similar
- Minimal changes in lipid parameters
Follow-up of dual therapy (PI/r + 3TC) arm at W96, N = 132
- Confirmed virological failure (2 consecutive HIV RNA > 500 c/mL)
- N = 8 (virological success = 94% [ HIV RNA < 50 c/mL: 79%])
- Genotype testing in 7/8: 2 lost M184V mutation, none developed PI or new NRTI mutations
- Reintroduction of TDF in 5/8 participants: 4/5 resuppressed, 1 data missing
- No change in 3 participants: resuppressed without change
- 3 discontinuations for non virological failure
- Global treatment success rate at W96: 91.7%
 Back to Table of Contents
Back to Table of Contents



