Objective
- Primary endpoint: proportion with HIV RNA < 50 c/mL at W24 and W48 (ITT, FDA snapshot) with non inferiority of DRV/r + RPV (lower limit of the 95% CI for the difference = -12%, 80% power)
- Protocol-defined virologic failure: 2 consecutive HIV RNA > 50 c/mL
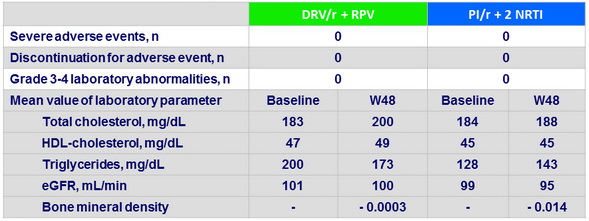
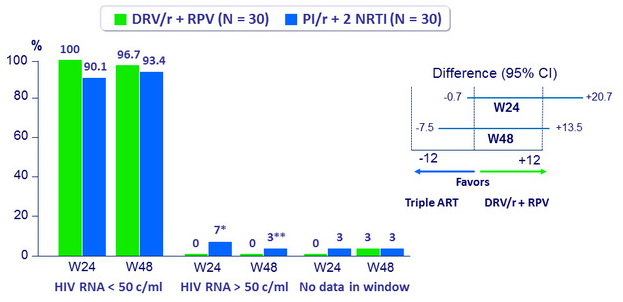
Baseline characteristics (mean)
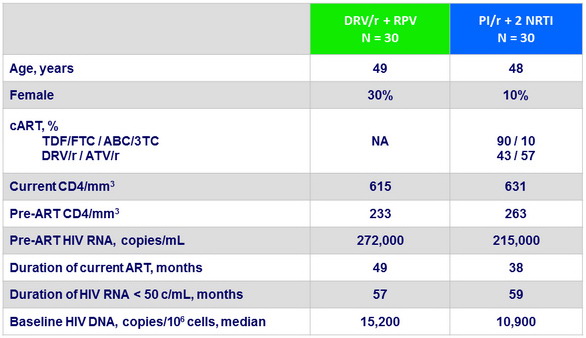
Virologic outcome at W24 and W48 (ITT, snapshot)
* blips at 57 and 138 c/mL
** blip at 59 c/mL
- Virologic non inferiority at W24 and W48
Secondary endpoints at W48
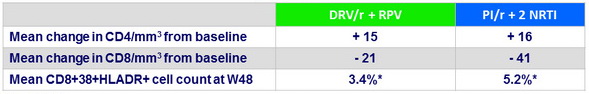
* p = 0.018
Safety and Tolerability at W48