Switch studies in virologically suppressed patients
Switch to ATV/ r + 3TC
SALT Study
Original article :
Perez-Molina JA. Lancet Infect Dis 2015; 15:775-84,
Perez-Molina JA,
JAC 2017; 72:246-53
Last update :
07/05/2018
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
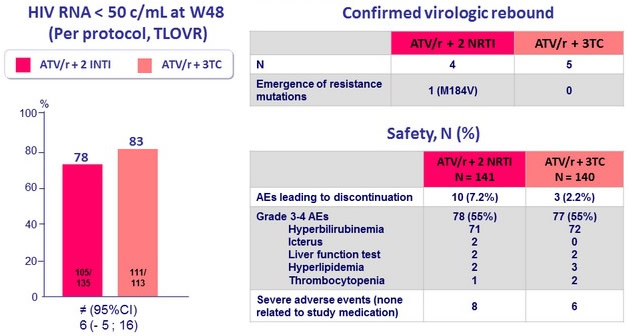
- Switching to ATV/r + 3TC is effective, safe, and non-inferior to ATV/r + 2 NRTI in virologically suppressed HIV+ patients, who need change any antiretroviral previous triple therapy because of toxicity, intolerance or simplification
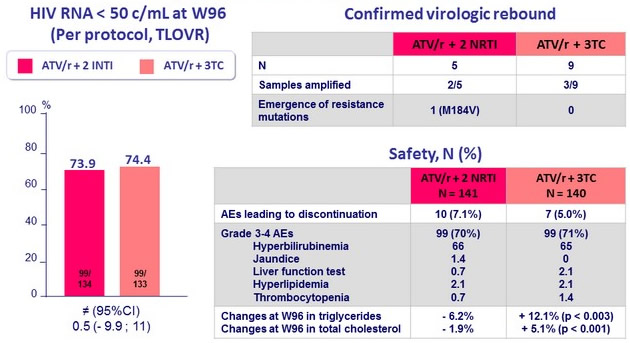
- Switching from a triple to a dual ATV-based regimen is not associated with an increased risk of virological failure, which was low in both groups, with most patients with virological failure with HIV RNA < 200 c/ml
- Frequency of blips throughout the 48 weeks was equivalent in both groups
- Only 1 patient (triple-treatment group) developed resistance mutation (M184V)
- Few patients discontinued the study in the 2 groups because of toxic effects, with treatment interruptions being significantly more frequent in the triple-treatment group
- No significant differences in change from baseline in neurocognitive function, neither renal function, bone mineral density, or fat gain or distribution between groups at week 96
Design
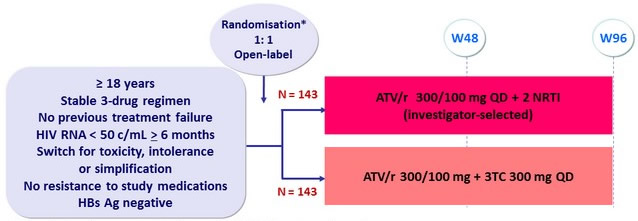
* Randomisation was stratified on active HCV infection and previous treatment
(NNRTI, PI/r, CCR5 antagonist, integrase inhibitor)
Objective
- Primary Endpoint : proportion with treatment success at W48
- Treatment failure : treatment discontinuation or modification for any cause or confirmed virologic rebound (2 consecutive HIV RNA > 50 c/mL )
- Non -inferiority of ATV/r + 3TC ( p er protocol) ; lower limit of the 95 % CI for the difference = -12%
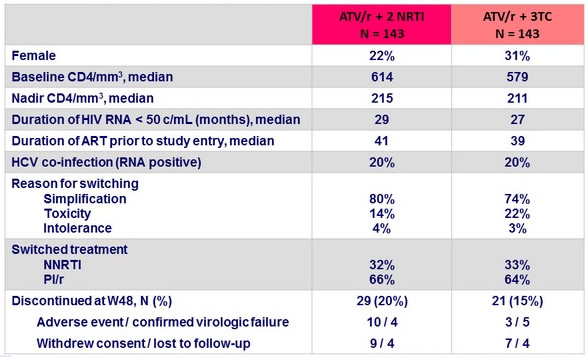
Baseline characteristics and disposition at W48
Efficacy and Safety Results (W48)
Efficacy and Safety Results (W96)
 Back to Table of Contents Back to Table of Contents
|



