Switch studies in virologically suppressed patients
Switch from TDF to TAF
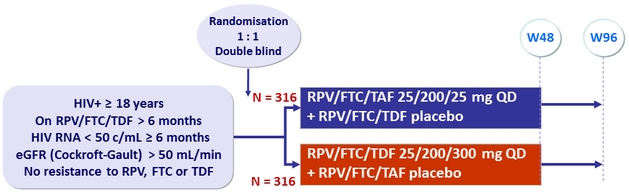
GS-US-366-1216 Study : Switch RPV/FTC/TDF
to RPV/FTC/TAF
Original article :
Orkin C. Lancet HIV 2017 ; 4:e195-204
Dernière mise à jour :
01/06/2017
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- Overall, virally suppressed, HIV-1 infected participants who switched to rilpivirine , emtricitabine, and tenofovir alafenamide maintained viral suppression at 48 weeks with low rates of virological failure, good tolerability, and improvements in measures of bone and renal safety compared with rilpivirine , emtricitabine, and tenofovir disoproxil fumarate
Design
Endpoints
- Primary: proportion of patients maintaining HIV RNA < 50 c/mL at W48 (ITT, snapshot) ; non-inferiority if lower margin of a two-sided 95.001% CI for the difference = - 8%, 85% power
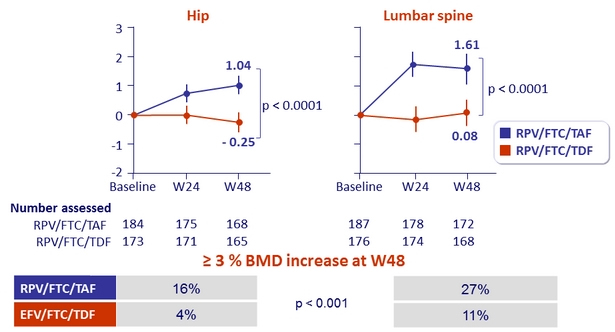
- Secondary: percentage change for hip and spine bone mineral density between treatment groups ; 90% power to detect a 1.38% difference (non-inferiority margin) ; multiple adjustments to test for superiority
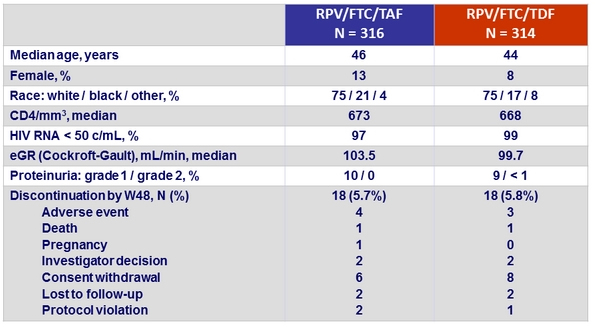
Baseline characteristics and outcome
Virologic outcome at W48 (ITT, snapshot)
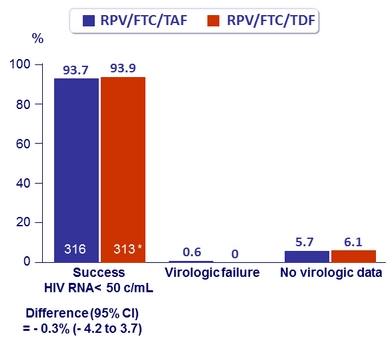
* 1 patient excluded from full- analysis set ( was taking EFV/FTC/TDF)
Other efficacy results at W48
- Per protocol analysis
(HIV RNA < 50 c/mL)
- ‒ 99.3% RPV/FTC/TAF ‒
- 100% RPV/FTC/TDF
- Virologic success was similar between treatment groups for the subgroups of age, sex, race, geographic region, and study drug adherence
- Mean changes in CD4/mm3
- ‒ + 9 RPV/FTC/TAF
- - 1 RPV/FTC/TDF
Resistance analysis
- Genotype and Phenotype testing if confirmed HIV RNA ≥ 50 c/mL
and confirmatory sample ≥ 400 c/mL, or HIV RNA ≥ 400 c/mL at W48
or at the last visit on study drug
- 1 patient in the RPV/FTC/TAF group: re-emergence of archived mutations M41K, E44D, D67N, V118I, L210W, T215Y; no new mutations (did not re-suppress)
- 1 patient in the RPV/FTC/TDF group: no resistance detected, was re-suppressed on continued therapy
- Historical genotypes: resistance mutations to study drug in 3 participants
- 3 patients in the TAF group: M184V (N = 2), E138A, K101E + E138K
- 1 discontinued at W4 with HIV RNA < 50 c/mL, 3 with HIV RNA < 50 c/mL at W48
- 3 patients in the TDF group: M184V, E138A (N = 2)
- All 3 with HIV RNA < 50 c/mL at W48
Adverse events, %
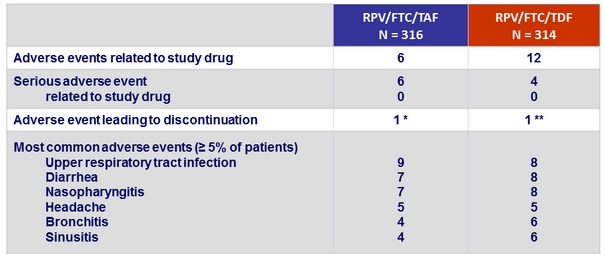
* Gastroesophageal reflux disease (N = 1), hiatus hernia and ulcerative oesophagitis (N = 1), fatigue
(N = 1), leading to discontinuation), suicidal depression (N = 1)
** Drug hypersensitivity (N = 1), leading to discontinuation), increased ALT and AST (N = 1), chronic myeloid leukaemia (N = 1)
Change in renal biomarkers at W48
Urine protein to creatinine ratio (% median change)
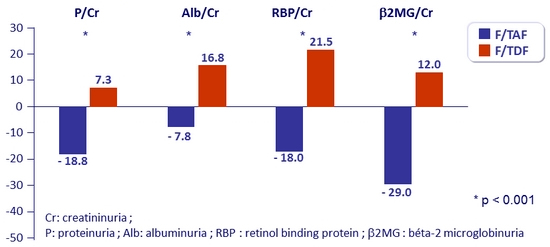
- Median change in eGFR : + 4.5 mg/ dL on RPV/FTC/TAF vs + 0.7 mg/ dL on RPV/FTC/TDF (p = 0.0024)
- No discontinuation for study-drug renal adverse event in either group
- No reported cases of proximal renal tubulopathy or Fanconi syndrome in either group
Mean % change in bone mineral density through W48 (%, 95% CI)
Fasting lipids changes at W48
- Increases in total cholesterol, direct LDL, HDL and triglycerides in the RPV/FTC/TAF group
- Stable in the RPV/FTC/TDF group
- Change in total cholesterol:HDL-cholesterol ratio: similar in both groups
- Introduction of lipid-lowering agent between baseline and W48: 4% in the RPV/FTC/TAF group vs 1% in the RPV/FTC/TDF group (p = 0.067)
 Back to Table of Contents Back to Table of Contents
|



