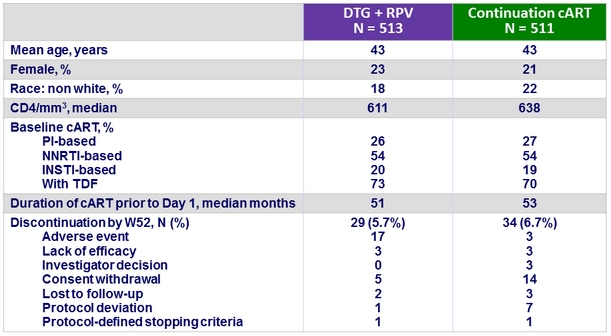
Baseline characteristics and patient disposition
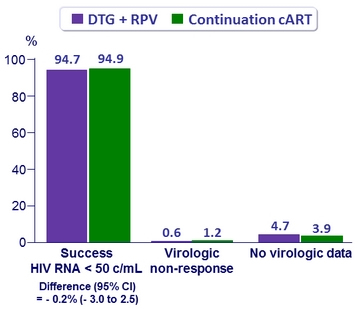
Virologic outcome at W48 (ITT-E, snapshot)
Other virologic results at W48
- HIV RNA < 50 c/mL (ITT-E snapshot)
- SWORD-1
- 95% DTG + RPV
- 96% continuation cART
- Adjusted ≠: - 0.6% (95% CI: - 4.3 to + 3.0)
- SWORD-2
- 94% DTG + RPV
- 94% continuation cART
- Adjusted ≠: 0.2% (95% CI: - 3.9 to + 4.2)
- Confirmed virologic failure:
HIV RNA ≥ 50 c/mL, retest ≥ 200 c/mL
- DTG + RPV, N = 2
- Emergence of NNRTI resistance mutation (K101K/E) ‒
- Continued cART , N = 2
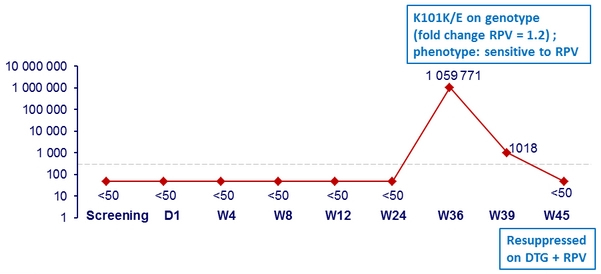
HIV RNA of subject with NNRTI- resistant mutation
- 41-year-old female
- Pre- cART HIV RNA > 2 millions c/mL ; 1 st cART : TDF/FTC/EFV
- Randomised to DTG + RPV
- Documented non-adherence before W36
HIV RNA, c/mL
Adverse events , %
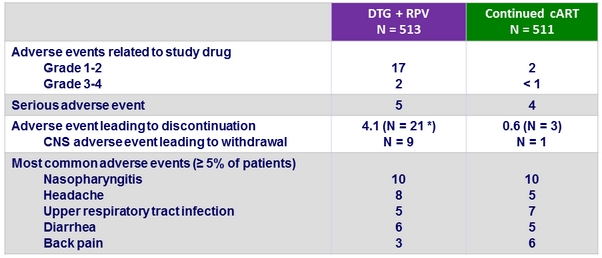
* (some participants have more than 1 AE) ; anxiety (N = 4), depression (N = 3), insomnia (N = 2), depressed mood (N = 1), headache (N = 1), panic attack (N = 1), suicidal ideation (N = 1), tremor (N = 1), drug-induced liver injury (N = 1), eosinophilic pneumonia, acute (N = 1), abdominal distension (N = 2), dyspepsia (N = 2), peptic ulcer (N = 1), gastrointestinal haemorrhage (N = 1), pancreatitis, acute (N = 1), Hodgkin's disease (N = 1), Kaposi sarcoma (N = 1), plasmablastic lymphoma (N = 1)
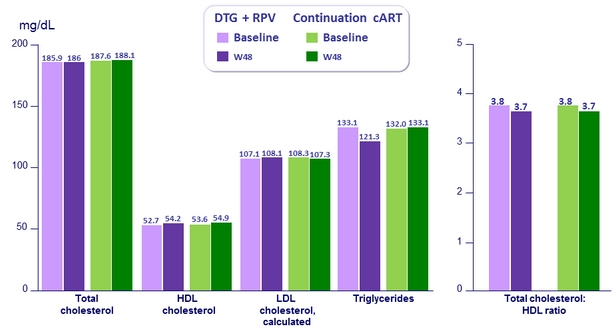
Fasting lipids at baseline and W48