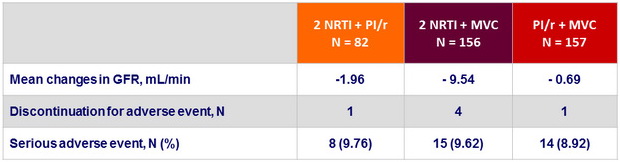
Baseline characteristics and disposition
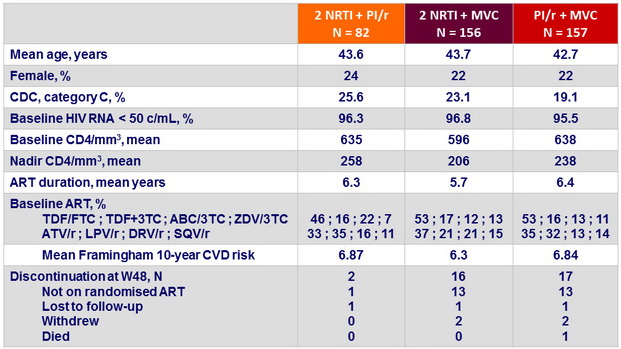
Virologic Outcomes – W48
* ≠ : - 4% ; (95% CI = - 9.0 to 2.2)
** ≠ : - 13.5% ; (95% CI = - 19.8 to -5.8)
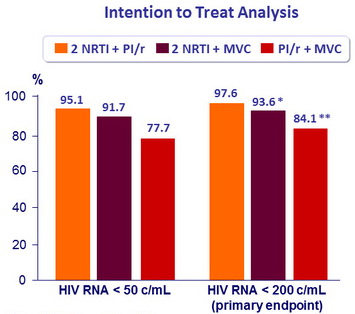
% with virologic response (HIV RNA < 200 c/mL), by week
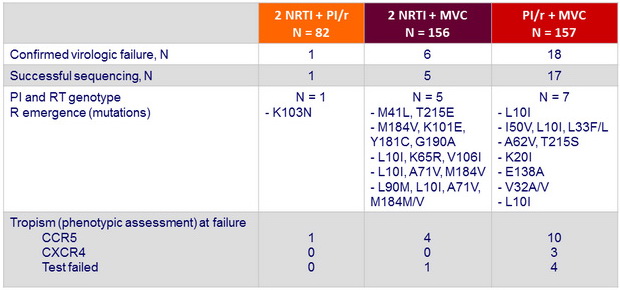
Emergent resistance in participants with confirmed virologic failure
Changes in immunologic and metabolic parameters
and quality of life over 48 weeks
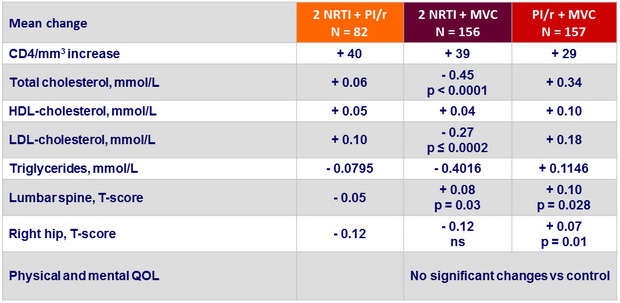
p : vs 2 NRTI + PI/r
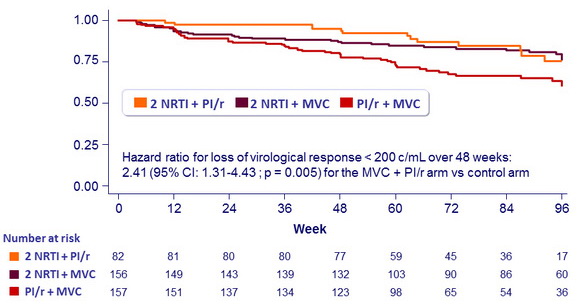
Safety at W48