Comparison of PI vs PI
Study ACTG A5073: LPV/r QD vs BID,�in combination with FTC + (d4T-XR or TDF)
Original article : Clin Infect Dis. 2010 Apr 1;50(7):1041-52. - C Flexner
Last update :
28/03/2014
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
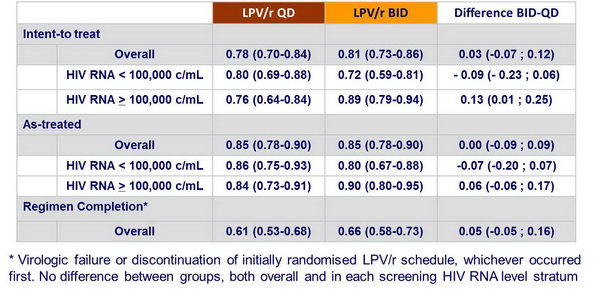
- Overall, LPV/r QD and BID had similar outcomes
- Patients with HIV RNA levels > 100,000 c/mL had better virologic response with LPV/r BID
Design :
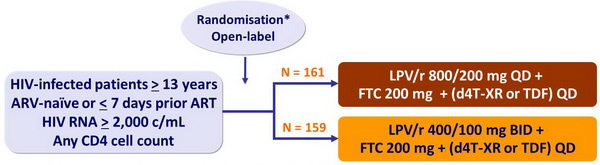
* randomisation was stratified on HIV RNA < 100,000 c/mL or ≥ 100,000 c/mL
The study had a third arm of LPV/r QD + FTC + (d4T-XR or TDF) administered by DOT in 82 patients, not included in the comparison of the 2 other arms
Objective :
- Primary endpoint: Kaplan-Meier of sustained virologic response at W48
- Sustained virologic response = lack of confirmed HIV RNA > 200 c/mL at W48 after confirmed HIV RNA < 200 c/ml ; or lack of confirmed HIV RNA > 200 c/mL at or after W24 ; lack of HIV RNA > 200 c/mL at W48 (no confirmation required)
- Width of < 0.2 for the 2-sided 95% CI for the difference in probability of SVR
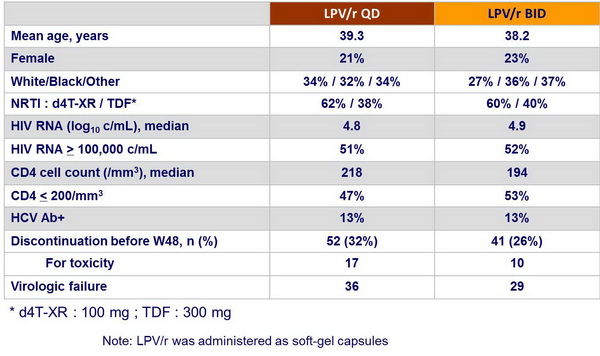
Patient disposition and baseline characteristics :
Estimated probability of sustained virologic response (95% CI) at W48 :
Emergence of resistance mutations to PI in virologic failure :
- Low incidence and no difference between QD (2/35 failure) and BID (3/26 failure)
Grade 3 or 4 clinical events and laboratory abnormalities :
- No significant difference in time to event between QD and BID
Adherence (electronic monitoring) :
- Significantly higher with QD during the first 24 weeks and between W24 and W48
Lopinavir Ctrough at week 16 and week 48 :
- Significantly lower and more variable with QD
- At W48, median Ctrough : 3.4 mg/mL for QD vs 5.6 mg/mL for BID
 Back to Table of Contents
Back to Table of Contents