Comparison of PI vs PI
ARTEMIS Study: DRV/r QD vs LPV/r (BID or QD),�in combination with TDF/FTC
Original article : AIDS. 2008 Jul 31;22(12):1389-97 - R Ortiz, AIDS. 2009 Aug 24;23(13):1679-88 - AM Mills, HIV Med. 2013 Jan;14(1):49-59 - C Orkin
Last update :
08/10/2014
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- DRV/r QD is non inferior to LPV/r, when co-administered with TDF/FTC(1)
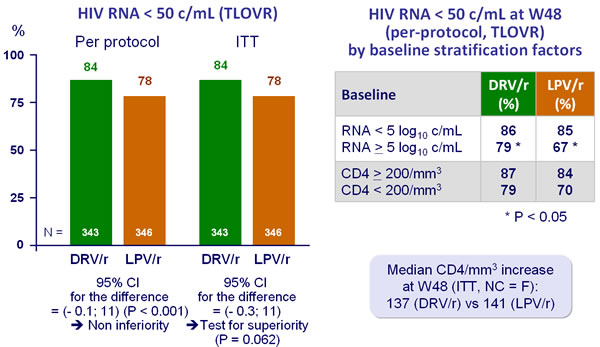
- Greater virologic response, at W48 (HIV RNA < 50 c/mL), of DRV/r�as compared with LPV/r in patients with high pre treatment HIV RNA (significant difference) or low CD4 count
- Lower incidence of diarrhoea with DRV/r vs LPV/r
- Lipid elevations were less pronounced with DRV/r
- Safety – W192 analysis
- Data similar to that seen at W96
- No new emerging AE with longer-term follow-up
- Grade 2-4 treatment-related diarrhoea was significantly less frequent with DRV/r than with LPV/r (5.0% vs. 11.3%, respectively; P = 0.003)
- DRV/r was associated with smaller median increases in total cholesterol and triglyceride levels than LPV/r. Changes in low- and high-density lipoprotein cholesterol were similar between groups
- Similar increases in aspartate aminotransferase and alanine aminotransferase for DRV/r and LPV/r were observed
Design :
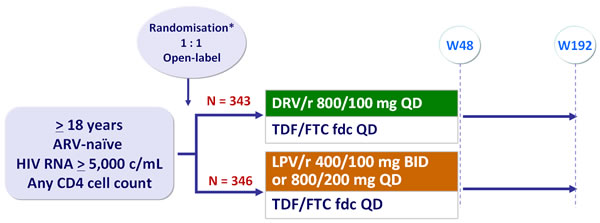
*Randomisation was stratified by HIV RNA (< or > 100,000 c/mL)�and CD4 (< or > 200/mm3) at screening
Objective :
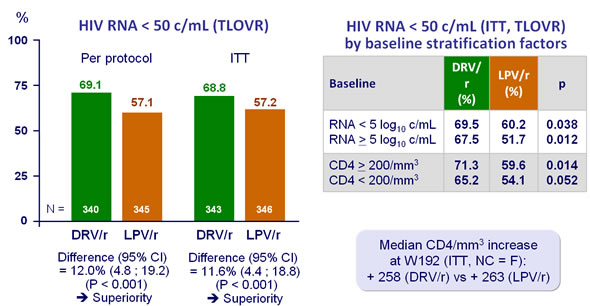
- Non inferiority of DRV/r vs LPV/r at W48: % HIV RNA < 50 c/mL by �per-protocol TLOVR analysis (lower margin of the 2-sided 95% CI for the difference = - 12%, 90% power). Superiority tested by ITT if non inferiority established
Baseline characteristics and patient disposition :
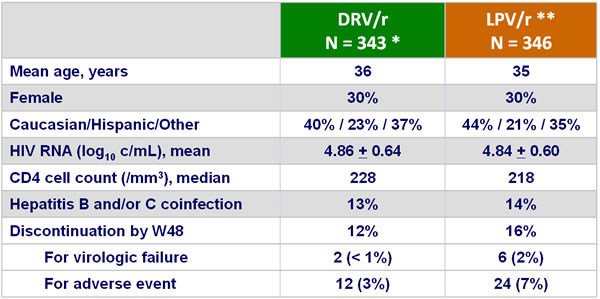
* 3 patients excluded for the per-protocol analysis (did not received study medication or received disallowed therapy for more than 1 week) ** LPV/r was administered BID or QD according to investigator and/or patient preference (77% received BID, 15% QD and 8% both; 15% received soft-gel capsules, 2% tablets and 83% switched from SGC to tablets)
Response to treatment at week 48 :
Virologic failure :
- Definition: HIV RNA never suppressed below 50 c/mL at W24 or confirmed HIV RNA > 50 c/mL after achieving < 50 c/mL or last observed HIV RNA > 50 c/mL followed by discontinuation
Resistance data :
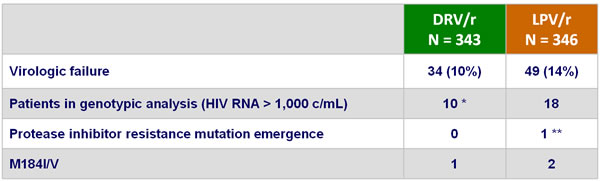
* 1 patient with HIV RNA > 1,000 c/mL did not have genotype available ** A71T and V77I
W48 Safety: DRV/r vs LPV/r :
- Discontinuations for adverse events (AE) were significantly less frequent in the DRV/r group: 3% vs 7% (P < 0.05)
- Rate of serious AE was not significantly different: 7% vs 12%
- Incidence of grade 2 to 4 gastrointestinal AE was significantly lower in the DRV/r group: 7% vs 14% (P < 0.01); these were mainly diarrhoea: �4% vs 10% (P < 0.01)
- Rash incidence was not significantly different: 3% vs 1%; 1 case of Stevens-Johnson occurred in the DRV/r group
- No patients discontinued because of renal events
- Mean increases in triglycerides and total cholesterol were less pronounced with DRV/r; grade 2 to 4 elevations in triglycerides and total cholesterol were significantly less frequent with DRV/r: 3% vs 11% �and 13% vs 23%, respectively
- Hepatic safety was similar in both groups
Summary – Conclusion (W48) :
- DRV/r QD is non inferior to LPV/r, when co-administered with TDF/FTC(1)
- Greater virologic response, at W48 (HIV RNA < 50 c/mL), of DRV/r�as compared with LPV/r in patients with high pre treatment HIV RNA (significant difference) or low CD4 count
- Lower incidence of diarrhoea with DRV/r vs LPV/r
- Lipid elevations were less pronounced with DRV/r
Overview at week 96 :
- At W96(2), significantly more DRV/r (79%) than LPV/r (71%) patients had HIV RNA < 50 c/mL confirming non inferiority and superiority (P = 0.012; ITT) in virologic response
- Safety outcomes confirmed W48 results: more favourable gastrointestinal and lipid profile of DRV/r QD
- Lipid-lowering agents use by W96: 8% LPV/r vs 7% DRV/r
- Overall, discontinuation for adverse events occurred in 4% of DRV/r patients vs 9% of LPV/r patients
Patient disposition at W96 and W192 :
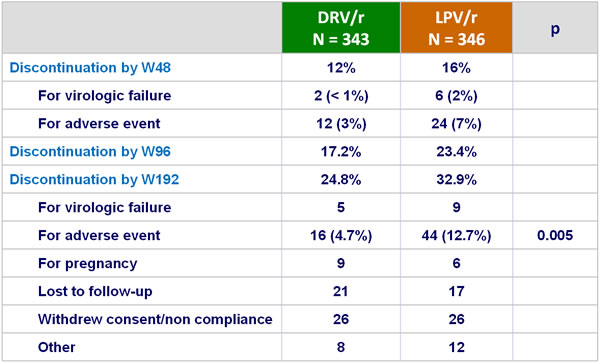
Final (week 192) analysis :
Resistance data at W192 :
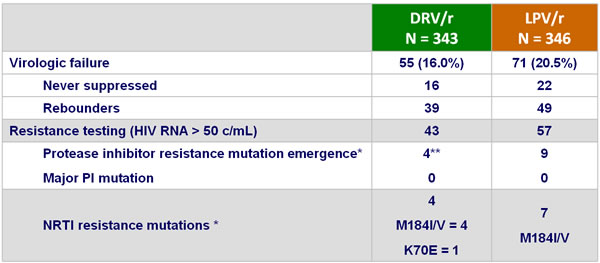
* At endpoint = last time point : with available genotype
** L10V, N = 1 ; V11I, N = 1 ; I13V, N = 1 ; I13V + G16E, N = 1
Safety – W192 analysis :
- Data similar to that seen at W96
- No new emerging AE with longer-term follow-up
- Grade 2-4 treatment-related diarrhoea was significantly less frequent with DRV/r than with LPV/r (5.0% vs. 11.3%, respectively; P = 0.003)
- DRV/r was associated with smaller median increases in total cholesterol and triglyceride levels than LPV/r. Changes in low- and high-density lipoprotein cholesterol were similar between groups
- Similar increases in aspartate aminotransferase and alanine aminotransferase for DRV/r and LPV/r were observed
 Back to Table of Contents
Back to Table of Contents