Head-to-head comparative trials for first line ART since 2006
Comparison of PI vs PI
KLEAN Study: FPV/r BID vs LPV/r BID,�in combination with ABC/3TC
Original article : Lancet. 2006 Aug 5;368(9534):476-82 - J Eron
Last update :
28/03/2014
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- In combination with ABC/3TC QD, FPV/r BID was non inferior to LPV/r BID
- Virologic and immunologic outcomes at W48 were similar with FPV/r and LPV/r
- In patients with high baseline HIV RNA and those with low baseline CD4, similar antiviral potency of the 2 PI/r was evidenced
- Tolerability and safety, numbers of treatment discontinuations, and increases in fasting lipids were similar for FPV/r and LPV/r
- Confirmed virologic failure was uncommon in both groups with no emergence of major protease inhibitor-associated resistance mutation in either group
- In antiretroviral-naïve patients, FPV/r BID provides similar antiviral efficacy, immunologic response, safety and tolerability as LPV/r, both in combination with fixed dose ABC/3TC QD
Design :
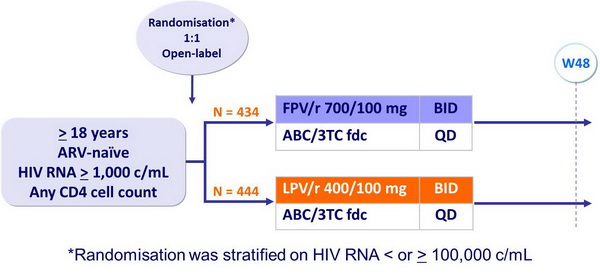
Objective :
- Non inferiority of FPV/r vs LPV/r at W48: % HIV RNA < 400 c/mL, ITT, TLOVR algorithm (lower margin of the 95% CI for the difference = - 12%, 90% power)
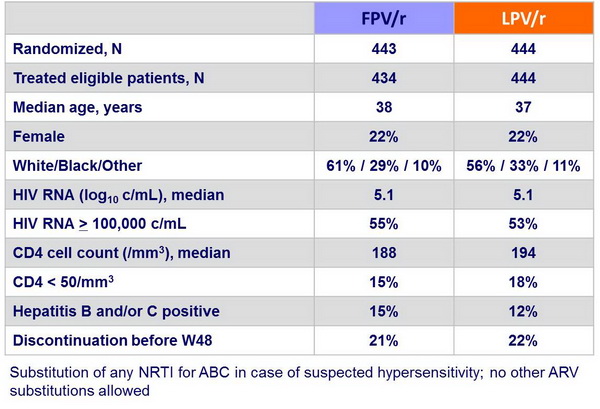
Baseline characteristics and patient disposition :
HIV RNA < cut-off at week 48 :
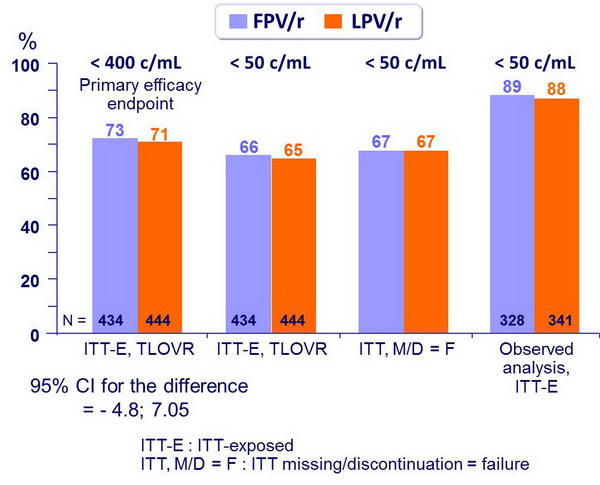
- % HIV RNA < 50 c/mL�(ITT-E, TLOVR) was�similar between FPV/r and �LPV/r across baseline�subgroups (low or high�HIV RNA, low or high CD4)
- Median CD4 increase�at W48: 176/mm3 (FPV/r)�vs 191/mm3(LPV/r)
- Virologic failures at W48 (TLOVR analysis): 26 (FPV/r) vs 30 (LPV/r), including unconfirmed HIV RNA �> 400 c/mL on final visit
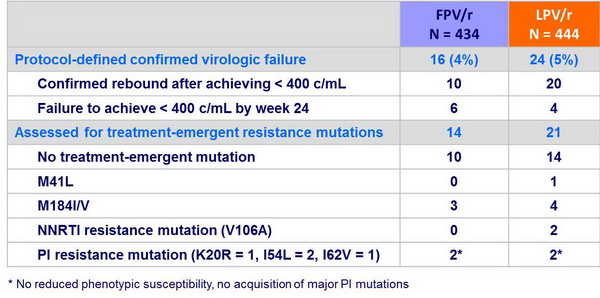
Resistance data :
- Genotypic and phenotypic resistance testing was done at virologic failure:
- Viral rebound (2 consecutives HIV RNA > 400 c/mL after achieving < 400 c/mL
- HIV RNA > 400 c/mL at W24
Safety and tolerability: FPV/r vs LPV/r :
- Similar frequency of premature discontinuations for adverse events: 12% vs 10%
- Similar frequency of clinical adverse events grade 2 to 4 and laboratory abnormalities grade 3 to 4 in both groups
- Diarrhoea was the most common adverse event, and led to treatment discontinuation in 1% and 2%, respectively
- Similar frequency of suspected abacavir HSR: 7% vs 5%
- Similar frequency of grade 3-4 alanine transaminase (ALT): 12% of patients with hepatitis B and/or C co-infection vs 1% in the absence of co-infection
- Similar changes in fasting lipids at W48, including triglycerides. Use of lipid-lowering agents during the study period: 11% in both groups
 Back to Table of Contents Back to Table of Contents
|