|
Head-to-head comparative trials for first line ART since 2006
Comparison of NRTI combos
Study ACTG A5202 : ABC/3TC vs TDF/FTC
Original article : N Engl J Med. 2009 Dec 3;361(23):2230-40 - PE Sax, CROI 2010. Abs. 59LB - ES Daar
Last update :
14/02/2014
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- For initial treatment of HIV-1 infection, patients with a screening HIV RNA > 100,000 c/mL whose regimen contains TDF/FTC as compared with ABC/3TC were significantly less likely
- to experience virologic failure: TDF/FTC superiority in virologic outcome was observed throughout the duration of the study and in multiple sensitivity analyses
- to experience tolerability failure
- Possible explanation: ABC/3TC is less potent than TDF/FTC
- Difference in virologic failure between NRTIs significantly increased with a lower�CD4 count
- Differences in virologic failure persisted after adjustement for multiple baseline covariates
- Occurrence of suspected hypersensitivity reactions did not influence study outcomes: equal number in both groups, virologic failure infrequent
- Important implications for clinical practice of this double-blind, randomized, prospective study
- Patients with high HIV RNA have a risk of virologic failure twice higher with ABC/3TC as compared with TDF/FTC
- Treatment guidelines recommend to consider results of this study when selecting NRTIs for first-line antiretroviral therapy in patients with high HIV RNA
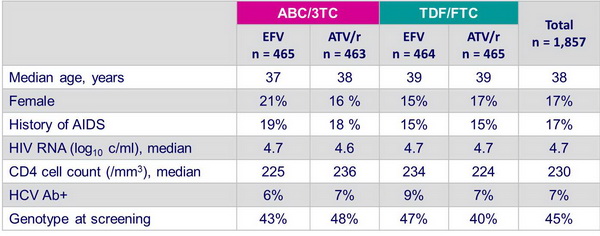
- Multicenter, randomized, blinded equivalence study in 1858 HIV-1 infected patients
- Comparison of antiviral activity, safety and tolerability of ABC/3TC and TDF/FTC, given with EFV or ATV/r
- Scheduled interim review by the DSMB of the NIAID: inferior virologic efficacy of ABC/3TC in patients with a screening HIV RNA > 100,000 c/mL
- Report of data from the 797 patients with screening
HIV RNA > 100,000 c/mL
- Inclusion criteria: HIV-1 infection, > 16 years, < 7 days of prior antiretroviral therapy, acceptable laboratory values
Design : randomized, partially blinded study comparing 4 once-daily regimens for the initial treatment of HIV-1 infection:
- EFV 600 mg or ATV/r 300/100 mg, in combination with ABC/3TC or TDF/FTC (double-blinding for the NRTIs),
- Randomisation was stratified on screening HIV RNA (> or < 100,000 c/mL),
- Planned study duration was 96 weeks after enrolment of the last patient,
- Genotypic resistance test was required in patients with recent HIV-1 acquisition,
- Testing for HLA-B*5701 was permitted but not required
Statistical analysis :
- Primary efficacy endpoint: time to virologic failure (confirmed HIV RNA > 1,000 c/mL at or after W16 and before W24, or > 200 c/mL at or after W24)
- Primary hypotheses:
- Equivalence of ABC/3TC and TDF/FTC (for each regimens with ATV/r and EFV)
- Equivalence of ATV/r and EFV (for each NRTI regimen)
- Equivalence if the two-sided 95% CI for the hazard ratio was between 0.71 and 1.40 (power of 89.8%)
- Pre specified early-stopping rules for inferiority at annual efficacy review by DSMB
- Analyses of efficacy by ITT, stratified according to the screening HIV RNA
- Kaplan-Meier estimation of time-to-event, with comparison by two-sided log-rank tests. Hazard ratios estimated by Cox models
- Primary safety endpoint: time to the first grade 3 or 4 sign, symptom, or laboratory abnormality at least 1 grade higher than at baseline (except isolated unconjugated bilirubin and creatine kinase) while on randomly assigned treatment
Baseline characteristics of the patients with screening HIV RNA > 100,000 c/mL :
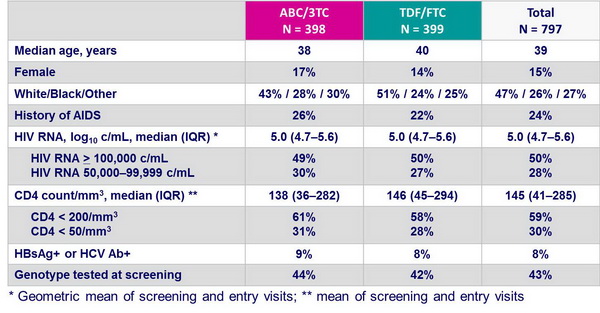
Time to virologic failure :
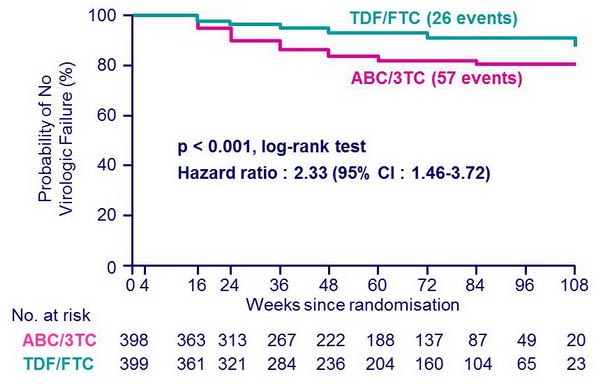
- Median follow-up = 60 weeks
- Discontinuation: 10% = 41 patients�on ABC/3TC and 38 on TDF/FTC
- Risk of subsequent virologic failure among 448 patients with > 2 consecutive HIV RNA < 50 c/mL = 12 in ABC/3TC group vs 9 in TDF/FTC group (p = 0.25)
- Median CD4/mm3 increase at W48:�194 (ABC/3TC) vs 199 (TDF/FTC)

Time to regimen failure* :
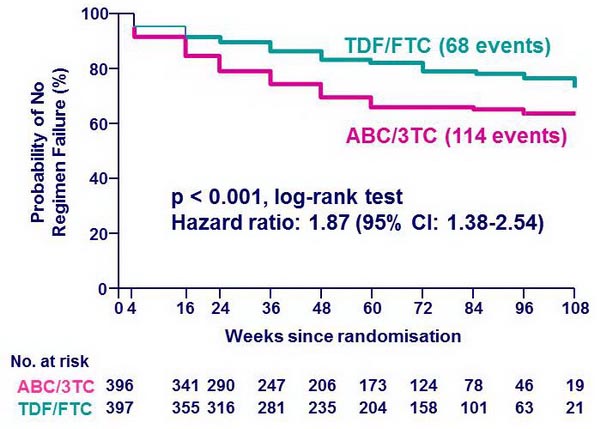
* Regimen failure: virologic failure or NRTI modification (time to first event)
Time to safety endpoint :
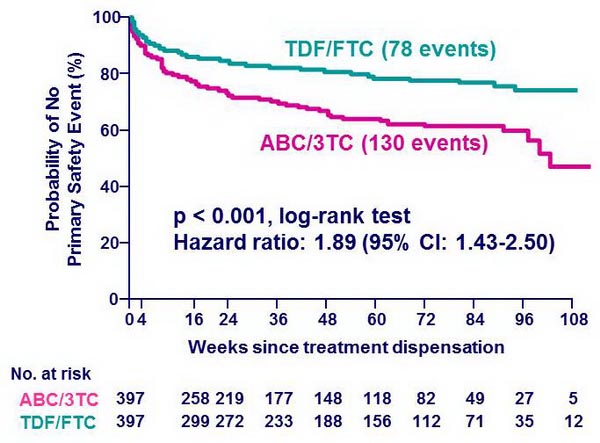
% of patients with HIV-1 RNA < 50 c/mL * :
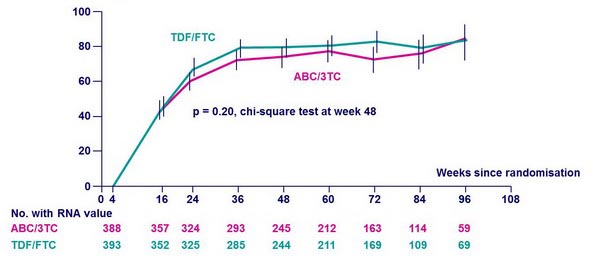
* ITT analysis involving all patients, regardless of prior NRTI discontinuation or virologic failure This analysis represents the aggregate success of both initial (randomly assigned) and � subsequent therapy
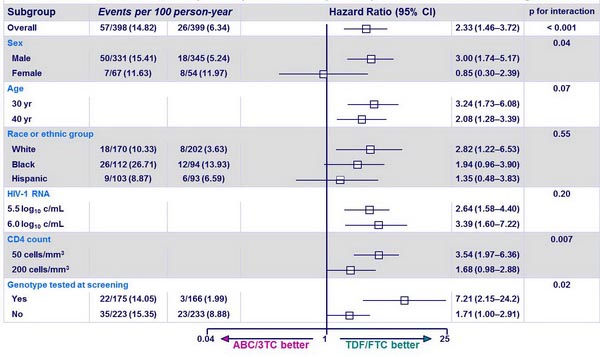
Estimated effect of ABC/3TC (N=398) vs TDF/FTC (N=399) on the hazard of virologic failure :
Grade 3 or 4 signs, symptoms or laboratory abnormalities at least �1 grade higher than the grade at baseline, during the initial regimen :
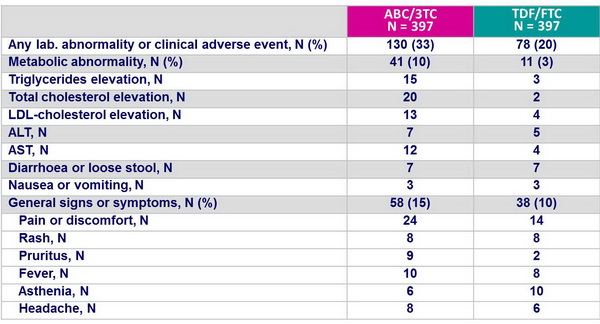
Selected clinical and laboratory events :
Patients characteristics at screenning - combination with EFV vs ATV/r - Final results (all patients) :
- Results in the 797 patients with screening HIV RNA > 100,000 c/mL :
- at DSMB action, time to virologic failure was significantly shorter with ABC/3TC as compared with TDF/FTC, independently of 3rd drug :
- [HR (95% CI)]: 2.33 (1.46-3.72) (Sax PE, NEJM 2009;361:2230-40)
- with EFV: 2.46 (1.20-5.05)
- with ATV/r: 2.22 (1.19-4.14)
Time to virologic failure - combination with EFV vs ATV/r - Final results (all patients) :
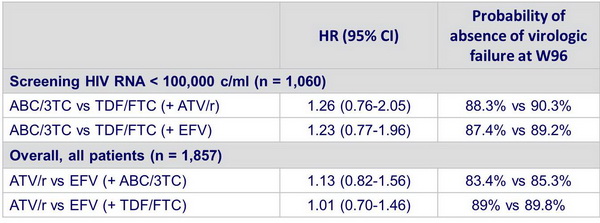
- In patients with screening HIV RNA < 100,000 c/ml, ABC/3TC and TDF/FTC �have similar time to virologic failure, with ATV/r and EFV
- Overall, ATV/r and EFV have similar time to virologic failure, with both NRTIs
Combination with EFV vs ATV/r - Final results (all patients) :
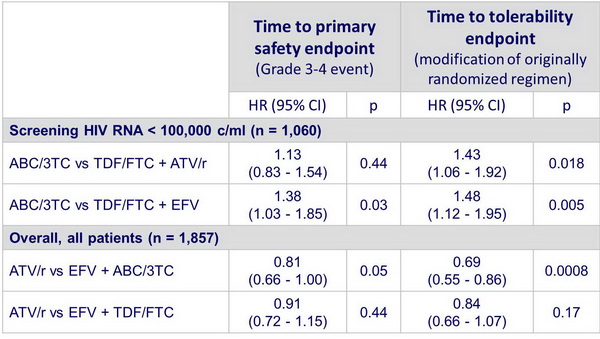
In patients with screening HIV RNA < 100,000 c/ml: ABC/3TC compared with TDF/FTC :
- Similar time to virologic failure with ATV/r and EFV
- Shorter time to safety event with EFV
- Shorter time to modification with ATV/r and EFV (difference driven by suspected hypersensitivity reactions in ABC/3TC arms)
- Greater increase in CD4 with EFV
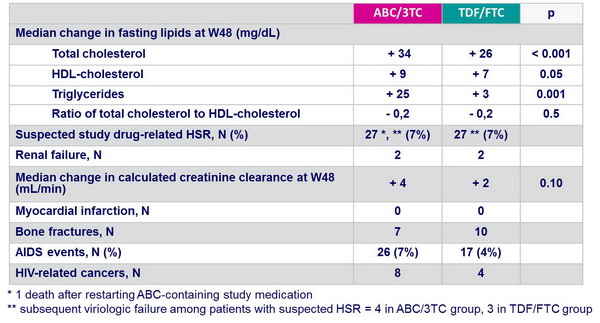
- Greater increase in total cholesterol, LDL- and HDL-cholesterol with both ATV/r and EFV; greater increase in triglycerides with ATV/r
- Increase (ABC/3TC) versus modest decline (TDF/FTC) in creatinine clearance with ATV/r
ATV/r compared with EFV (all patients) :
- Similar time to virologic failure with both NRTIs:
- Pre-specified equivalence boundary on HR was not met, as observed W96 event rate was lower than projected (~15% vs 32%),
- Difference and CIs for probability of being failure free at W96 were within + 10% criteria often used for defining equivalence (post hoc analysis)
- Longer time to safety event and to 3rd drug modification with ABC/3TC
- Among virologic failures there was less resistance with both NRTIs
- Greater increase in CD4 with TDF/FTC
- Smaller increases in total cholesterol, LDL- and HDL-cholesterol with both NRTIs
- Modest decline in creatinine clearance with TDF/FTC vs increase with ABC/3TC
 Back to Table of Contents Back to Table of Contents
|