Design
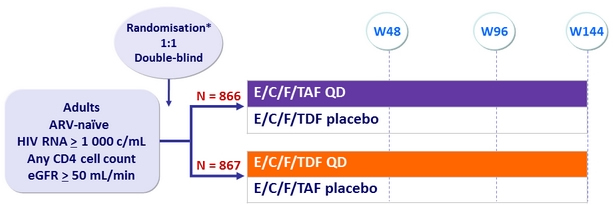
* Randomisation was stratified by HIV RNA (≤ or > 100,000 c/mL), CD4 cell count at screening,
and geographic region
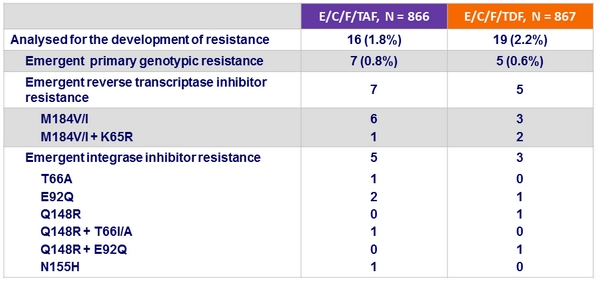
Criteria for resistance testing
- 2 consecutive HIV RNA ≥ 50 c/mL (with the second ≥ 400 c/mL ) after achieving < 50 c/mL , or HIV RNA ≥ 400 c/mL at W48 or last study visit
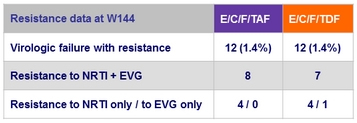
Resistance data at week 48
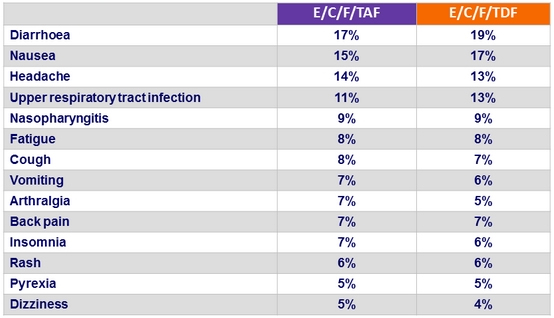
Adverse events (all grades) occurring in ≥ 5% of patients
in either group (W48)
Adverse events leading to study drug discontinuation
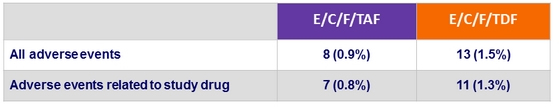
Deaths
Grade 3 or 4 laboratory abnormalities in ≥ 1% of patients in either group (W48)
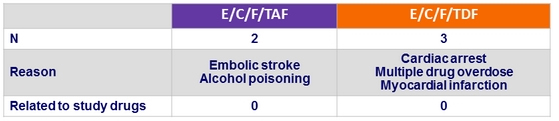
Discontinuation for renal event
- E/C/F/TAF = 0
- E/C/F/TDF = 4 : renal failure = 2, decreased GFR = 1, nephropathy = 1
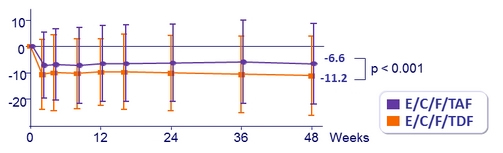
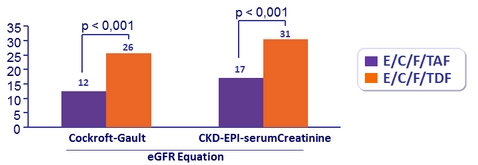
Mean (SD) change in eGFR (Cockcroft-Gault), mL /min, from baseline
Patients with ≥ 25% Decrease in eGFR
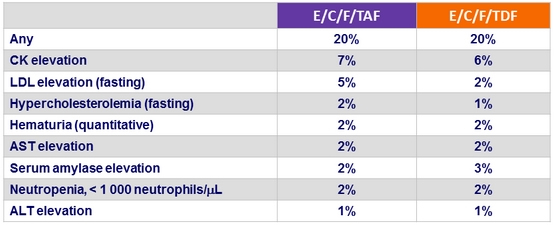
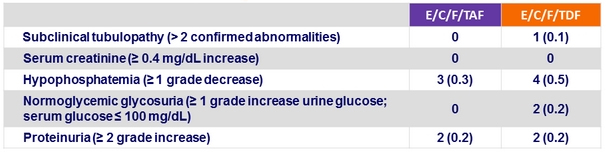
Renal laboratory abnormalities at week 48, n (%)
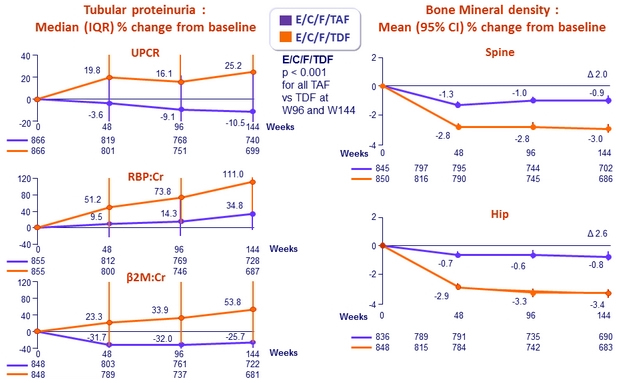
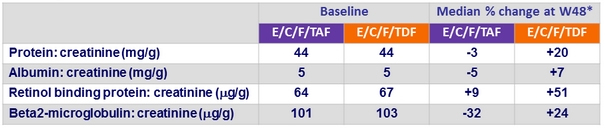
Changes in Quantitative proteinuria/creatininuria ratio at week 48
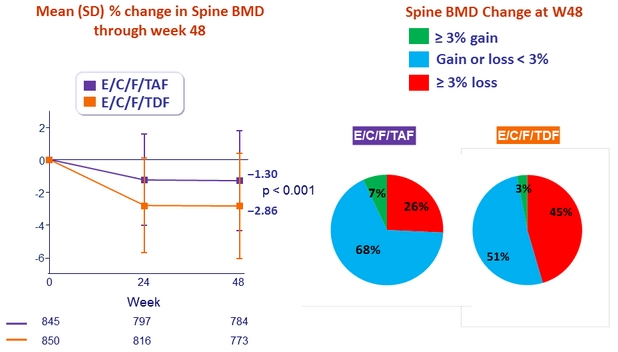
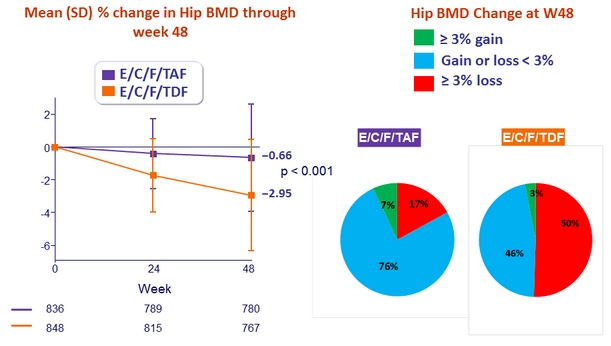
* p < 0.001 for all
Fasting Lipids at Week 48
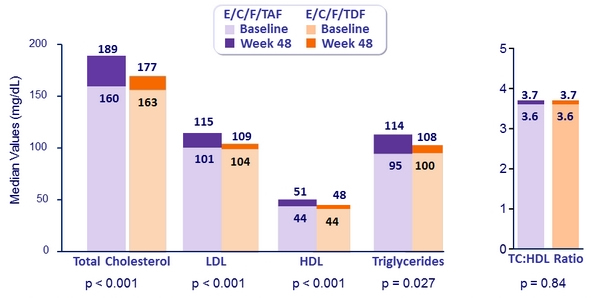
Patients initiating lipid-modifying medications: 3.6% E/C/F/TAF vs 2.9% E/C/F/TDF (p = 0.42)
HIV RNA < 50 c/mL at Week 96 and W144
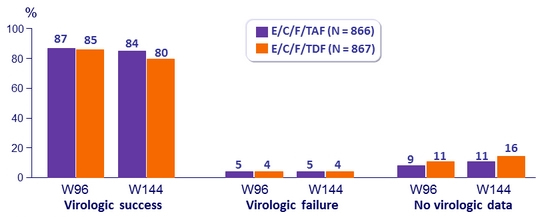
Difference in virologic success (95% CI)
- W96: 1.5% (-1.8 to 4.8)
- W144: 4.2% (0,6 to 7,8) ; p = 0.02
Renal events leading to treatment discontinuation at W144
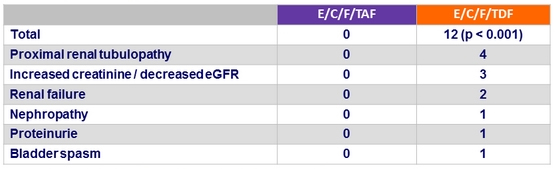
Adverse events leading to treatment discontinuation at W144
- 1.3% E/C/F/TAF vs 3.3% E/C/F/TDF (p = 0.01)
Grade 3-4 adverse events
- No difference between both groups at W144, except for
- LDL-cholesterol increase: 11.0% E/C/F/TAF vs 4.8% E/C/F/TDF
- Total cholesterol increase: 4.7% vs 2.8%
- Lipase increase: 5.0% vs 8.0%
- Amylase increase: 2.6% vs 5.0%