Comparison of NRTI combos
Study ASSERT : ABC/3TC vs TDF/FTC
Original article : J Acquir Immune Defic Syndr. 2010 Sep;55(1):49-57 - FA Post, Antivir Ther. 2013;18(7):905-13 - GJ Moyle, Clin Infect Dis. 2010 Oct 15;51(8):963-72 HJ Stellbrink
Last update :
08/10/2014
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- No difference in estimated glomerular filtration rate between ABC/3TC + EFV and TDF/FTC + EFV
- However, increases in markers of tubular dysfunction in the TDF/FTC arm
- No differences in markers of glomerular dysfunction over 96 weeks
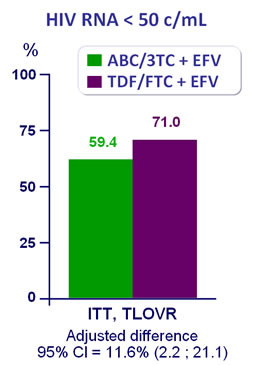
- Significant higher virologic efficacy for TDF/FTC + EFV
- Emergence of resistance to NNRTI and NRTI on virological failure with ABC/3TC + EFV but not with TDF/FTC + EFV
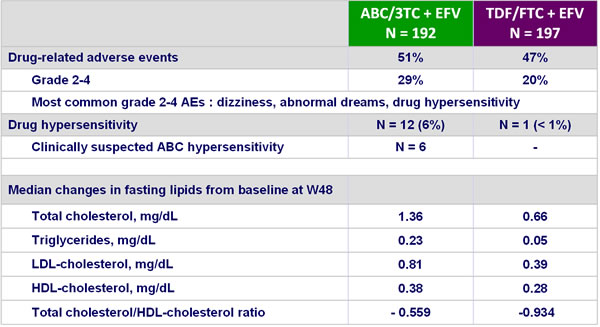
- Incidence of grade 4 adverse events and serious adverse events was higher in the ABC/3TC arm, mainly because of drug hypersensitivity or hypersensitivity
- Smaller increases in serum lipids with TDF/FTC
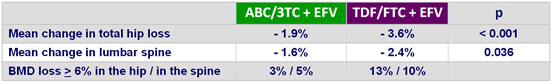
- Greater increases in bone turnover and decreases in hip and vertebral bone mineral density with TDF/FTC + EFV compared to ABC/3TC �+ EFV
Design :
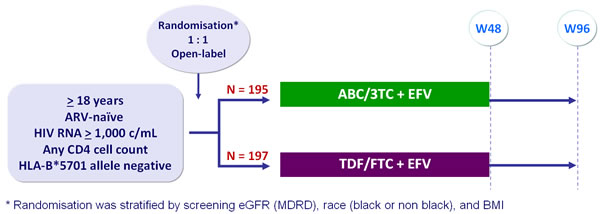
Primary analysis :
- Intention to treat, exposed analysis : 2-sided significance level of 5%, with 90% power to detect a difference of 10 mL/min in eGFR (MDRD) change from baseline between arms
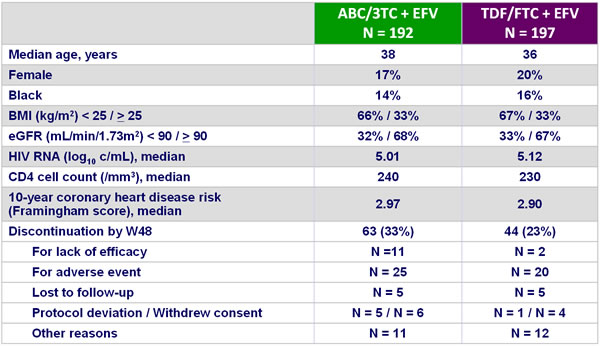
Baseline characteristics and patient disposition :
Adjusted mean change from baseline in eGFR by MDRD �(ml/min/1.73 m2),
ITT-e population :
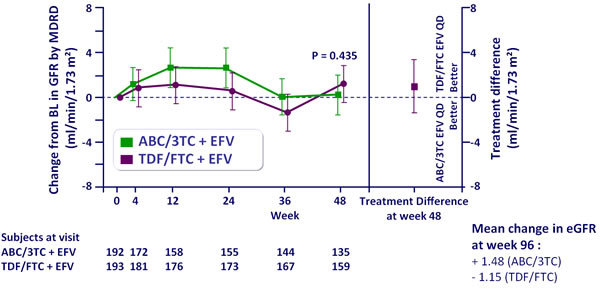
Repeated measures mixed model analysis
Secondary renal endpoints :
- No differences between arms in proportion with significant declines in eGFR �or with renal failure
- Percentage change from baseline at week 48
- Urinary retinol-binding protein/creatinine ratio : TDF/FTC = + 50% vs ABC/3TC = 0% (p < 0.0001)
- Urinary b2-microglobulin/creatinine ratio : TDF/FTC = + 24% vs ABC/3TC = - 47% �(p < 0.0001)
- No proximal renal tubular dysfunction over 96 weeks
Changes in bone density (DXA) at week 48 :
Bone turnover markers : increased in both groups over the first 24 weeks (significantly greater with TDF/FTC), stabilizing or decreasing thereafter
Efficacy at week 48 :
 Protocol-defined virologic failure (< 1 log10 c/mL reduction in HIV RNA by W4, confirmed rebound ≥ 400 c/mL after confirmed HIV RNA < 400 c/mL by W24 or confirmed HIV RNA > 400 c/mL after W24)
Protocol-defined virologic failure (< 1 log10 c/mL reduction in HIV RNA by W4, confirmed rebound ≥ 400 c/mL after confirmed HIV RNA < 400 c/mL by W24 or confirmed HIV RNA > 400 c/mL after W24)
- 6 on ABC/3TC + EFV
- Emergence of resistance : 3/6
- 2 patients with NNRTI mutations
- 1 patient with NNRTI mutation and K65R + D67N
- 2 on TDF/FTC + EFV = no resistance emergence
Median CD4/mm3 increase at W48 : + 150 in both groups
Safety at W48 :
 Back to Table of Contents
Back to Table of Contents



