BMS-955176 (maturation inhibitor)
AI468002 Study : BMS-955176 Phase II
Original article :
Hwang C. CROI 2015, Abs. 114LB
Last update :
15/05/2015
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK
- Virologic response rates (mITT and observed) and immunologic responses were similar across the BMS-663068 and ATV/r arms through Week 48
- All BMS-663068 doses were generally well tolerated with no dose-response safety signals reported
- Continuation dose of BMS-663068 1200 mg QD for the Phase IIb study
- Phase III study in heavily treatment-experienced patients with limited therapeutic options
- Phase III dose : 600 mg BID
- Subjects enrolled regardless of baseline susceptibility to BMS-626529
- A retrospective analysis will be conducted to determine whether a baseline phenotypic assay is necessary in the future
Design :
- Phase IIa , randomised, double- blind , dose- escalating study
- ARV-naïve (≤ 1 week of treatment) or experienced (PI and maturation inhibitor naïve ) patients, ≥ 18 years, HIV RNA > 5,000 c/mL, CD4 cell count > 200/mm3
- For all dose groups : 8 patients on BMS-176 qd and 2 on placebo
- HIV RNA evaluated at Day 1 to 14, Day 17-19 and Day 24

Baseline characteristics
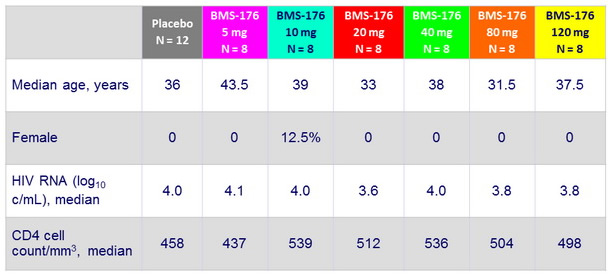
ARV-naïve : 92% ; ARV- experienced : 8%
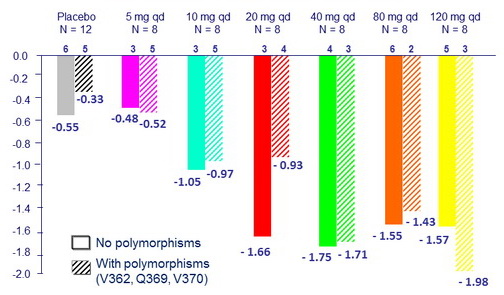
Maximum median reduction in HIV RNA from baseline, log10 c/mL
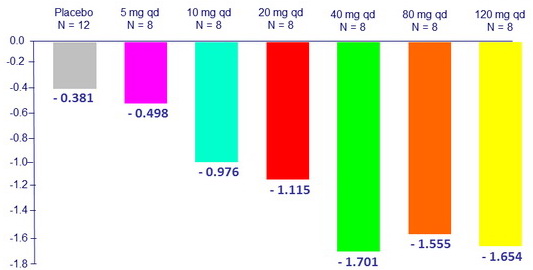
- Median change in HIV RNA from baseline to day 11 was – 1.4 log10 c/ mL
- BMS-955176 exposure-response relationship is consistent with dose-response antiviral activity
Maximum median reduction in HIV RNA, log10 c/mL
by baseline Gag polymorphisms
Adverse events
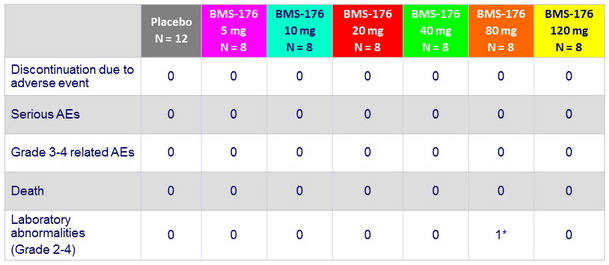
* Transient grade 3 neutropenia reported as related to study drug
 Back to Table of Contents
Back to Table of Contents



